Введение
С момента открытия вируса гепатита С стала очевидной определенная связь между персистированием хронического вирусного гепатита С (ХГС) и поражением почек, которое относится к одному из наиболее значимых системных клинических проявлений течения ХГС и имеет существенное значение для клинической нефрологической практики [1–3]. Так, было показано явное преобладание антител к вирусному гепатиту С (ВГС) у лиц с патологией почек по сравнению с донорами крови в регионах как с высокой, так и с низкой распространенностью ХГС, а также у лиц с острыми и хроническими нефропатиями после трансплантации почки [3–5].
Кроме того, у пациентов с ХГС значительно чаще имеют место реакции отторжения почечного трансплантата, развитие мембранознопролиферативного гломерулонефрита и мембранозной нефропатии [4–6]. Имеются данные о большей распространенности и гломерулярных, и тубулоинтерстициальных повреждений у пациентов с ХГС ассоциированными формами гломерулопатий [6, 8, 9].
Явную ассоциацию ХГС с развитием гломерулопатий подтверждают морфологические исследования, указывающие на высокую распространенность поражений клубочков в этой популяции пациентов [5, 7, 9]. Наконец, связь между ХГС и поражением почек также подтверждается и наблюдениями о положительной динамике почечных симптомов на фоне успешной противовирусной терапии – ПВТ [10–12].
У пациентов отделений хронического гемодиализа отмечается низкий уровень иммунной защиты, что служит причиной стертого течения вирусных гепатитов В и С [1, 13, 14]. В связи с этим многие больные центров хронического гемодиализа переносят малосимптомные формы как острых, так и хронических форм заболевания. На высокую частоту хронизации вирусных гепатитов у больных в терминальной стадии хронической почечной недостаточности (ХПН) указывает большинство исследований (F. Fabrizi et al., 2000; F. Fabrizi, F. Lokatelli, 1999; K. Ocuda et al., 1998).
T. Morisava et al. (1999) объясняют малосимптомное течение ХГС у больных в терминальной стадии ХПН тем, что эти пациенты систематически получают лечение гемодиализом, в результате чего происходит регулярное удаление вирусов и продуктов их жизнедеятельности из кровеносного русла, а следовательно, снижается уровень виремии [15, 16].
Отдельной проблемой служит лечение больных ХПН, инфицированных вирусами гепатитов В и С. Большинство исследователей связывают эффективность ПВТ у больных ВГС с генотипом вируса (N.N. Zein, 2000; G. Ballardini et al., 1997; M.U. Mandelli et al., 1997).
Классические схемы ПВТ с использованием препаратов (ПЕГ)-интерферона и рибавирина сопряжены с множеством осложнений. Однако в связи с необходимостью дозировать рибавирин в зависимости от скорости клубочковой фильтрации (СКФ), риском необратимого ухудшения функции почек, а также побочными эффектами интерферона данный режим рекомендован в руководствах с оговорками [2, 8]. При этом частота достижения устойчивого вирусологического ответа (УВО) при данном лечении составляет примерно 40–60%. В связи с этим проведение безинтерфероновой ПВТ особенно обосновано для данной категории пациентов.
В отечественной литературе практически отсутствуют данные о применении препаратов интерферона больными вирусным гепатитом в терминальной стадии ХПН, находящимся на программном гемодиализе.
Материал и методы
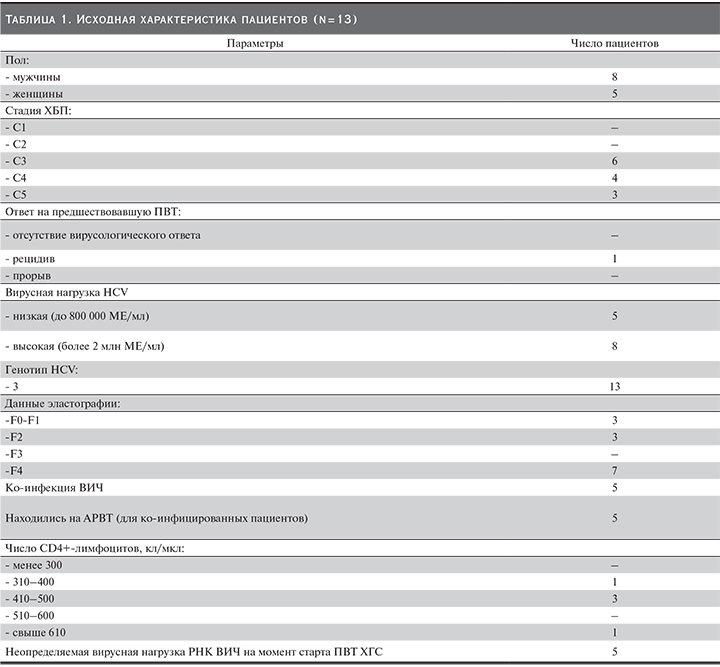
Группа пациентов ВГС+хроническая болезнь почек (ХБП) включала 13 пациентов, преимущественно мужчин (8 человек). Средний возраст составил 48±3,14 года.
Характеристика пациентов представлена в табл. 1.
При объективном обследовании у наших пациентов до начала ПВТ в клинической картине преобладали признаки астеновегетативного синдрома, также отмечались жалобы со стороны желудочно-кишечного тракта: снижение аппетита, тяжесть или боли в правом подреберье, неустойчивый стул.
Гепатомегалия была выявлена у шести пациентов, спленомегалия – у четырех.
При анализе клинически значимых биохимических параметров крови у 80% пациентов выявлено наличие цитолитического синдрома. Активность аланинаминотрансферазы (АЛТ) составляла 57–300 МЕ/мл.
Один пациент получал ранее комбинированную ПВТ ХГС пегилированными интерферонами в сочетании с рибавирином в течение 24 недель. У него развился рецидив через 24 недели после окончания лечения.
В 2018 г. в рамках программы «раннего доступа» по жизненным показаниям всем пациентам проводилась ПВТ с использованием схемы «глекапревир+пибрентасвир» в течение 8 недель (6 пациентов) и 12 недель (7 пациентов). Длительность курса ПВТ определена в соответствии с инструкцией к препарату: 12-недельный курс назначен пациентам с выраженным фиброзом печени (F4 по Metavir).
Исследуемый режим представляет собой комбинацию двух пангенотипных противовирусных препаратов прямого действия в фиксированных дозах: глекапревир 100 мг (ингибитор протеазы NS3/4A) и пибрентасвир 40 мг (ингибитор NS5A), воздействующих на различные этапы жизненного цикла вируса гепатита С.
В проведенных ранее исследованиях эффективности и безопасности данной схемы лечения пациентов с нарушением функции почек (EXPEDITION-4) и пациентов с ко-инфекцией ВИЧ (EXPEDITION-2) отмечены высокая эффективность данного препарата, а также хорошая его переносимость, редкие и незначительные отклонения в лабораторных показателях. Данная схема не требует коррекции дозы у особых групп пациентов.
Все 13 пациентов, получивших лечение в рамках программы раннего доступа, страдали ХБП разных стадий. Общие клинические проявления поражения почек у пациентов варьировались от бессимптомных изменений в анализах мочи (протеинурии, микрогематурии) до развития быстропрогрессирующего нефритического синдрома и повреждения почек ренального характера, требовавших проведения программного гемодиализа (7 пациентов).
Развитию гломерулопатий у всех наших пациентов клинически соответствовало появление существенной протеинурии (>1 г/сут), гематурии, нефритического и нефротического синдромов. Только у 7 больных отмечена развернутая клиническая картина гломерулярного поражения, у 2 из них подтвержденного морфологически, во всех случаях с развитием протеинурии >1 г/сут. У всех пациентов лабораторно была выявлена криоглобулинемия. У 5 из них отмечено повышение систолического и диастолического артериального давления и анемия легкой степени.
Двум пациентам с HCV-связанным криоглобулинемическим гломерулонефритом в анамнезе иммуносупрессивная терапия проведено лечение ритуксимабом в виде четырех еженедельных доз по 375 мг/м2.
У всех пациентов с ко-инфекцией ВИЧ была установлена стадия 4А-ремиссия (В.В. Покровский, 2001). В структуре сопутствующих заболеваний у них преобладали хроническая герпетическая инфекция, грибковые заболевания, в т.ч. кандидозы и латентное течение цитомегаловирусной инфекции. Диагноз ВИЧ-инфекции был установлен в среднем 8±2,6 года назад.
Все пациенты на момент старта терапии препаратом «глекапревир+пибрентасвир» получали АРВТ в среднем в течение 3±1,2 года, вирусная нагрузка ВИЧ была ниже порога определения. На период проведения ПВТ с учетом межлекарственных взаимодействий все пациенты были переведены на схему АРВТ, содержавшую ралтегравир: ламивудин+абакавир/или тенофовир+ралтегравир.
Результаты исследования
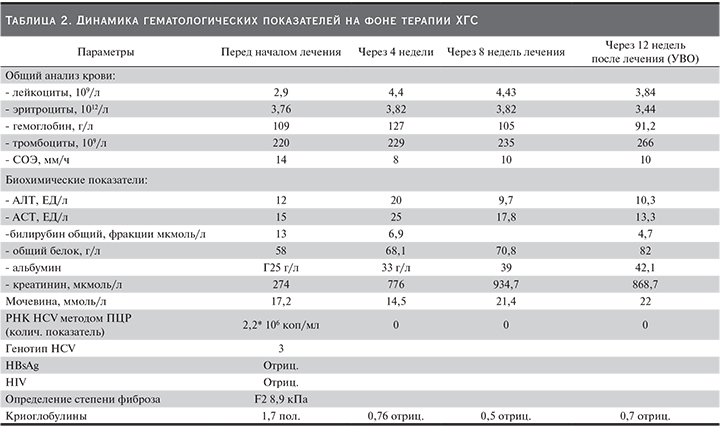
У всех 13 пациентов зафиксирован непосредственный вирусологический ответ. Стойкий вирусологический ответ также был достигнут всеми пациентами (УВО 100%).
Декомпенсаций печеночных функций не было. Летальные исходы за период наблюдения зарегистрированы не были.
У 10 пациентов произошла нормализация показателей АЛТ после окончания ПВТ. У трех пациентов с ко-инфекцией ВИЧ сохранились повышенные уровни АЛТ и АСТ (не более двух норм), что значительно ниже данных показателей до старта ПВТ ХГС.
После проведения ПВТ у 75% пациентов отмечено снижение протеинурии, уровня криоглобулинов. Кроме того, у пациентов отмечено достоверное повышение уровня альбумина сыворотки. Напротив, уровень креатинина сыворотки крови остался стабильным.
Обсуждение
Переносимость терапии в целом была удовлетворительной. У 1 пациента 53 лет, находившегося на программном гемодиализе с 1997 г., на 10-й неделе ПВТ возник побочный эффект в виде инфекции сосудистого доступа для проведения программного гемодиализа, сопровождавшейся повышением температуры тела до 400С, что потребовало госпитализации. В анамнезе этот пациент перенес 3 подобных эпизода инфекции сосудистого доступа (2001, 2006, 2012), внебольничную пневмонию (2014). Побочные эффекты не были связаны с проведением ПВТ. Пациенту провели оперативное лечение – сняли протез артериовенозной фистулы на левом плече, также проведена антибактериальная и дезинтоксикационная терапия. Нежелательное явление закончилось выздоровлением и, несмотря на сокращение сроков ПВТ до 10 недель, от данного пациента также получен УВО.

В заключение демонстрируем клинические примеры эффективности и безопасности ПВТ ВГС у пациентки с хроническим поражением почек и у пациентки с ко-инфекцией ВИЧ/ВГС и ХБП.
Клинический пример 1
Больная А. 39 лет наблюдается с 2016 г. с диагнозом «хронический гломерулонефрит, экстракапиллярный с 73% клеточных и фиброзноклеточных полулуний, ассоциированный с вирусным гепатитом С. ХБП 5Д-ст.». Программный гемодиализ с 04.2017. Диагноз ХГС поставлен в 2010 г., ПВТ не получала. В 2016 г. на фоне выраженного нефротического синдрома установлен диагноз «хронический гломерулонефрит, экстракапиллярный с 73% клеточных и фиброзноклеточных полулуний, ассоциированный с вирусным гепатитом С (подтвержден морфологически)». Проведено 2 курса пульс-терапии глюкокортикостероидами без эффекта, с 04.2017 3 раза в неделю проводили программный гемодиализ.
Данные объективного осмотра на момент назначения ПВТ: состояние удовлетворительное, кожа чистая, бледная. Лимфоузлы не увеличены, безболезненные. Зев умеренно гиперемирован, на слизистой оболочке полости рта налета нет. В легких дыхание везикулярное, тоны сердца ясные, ритм правильный. Живот мягкий, умеренно болезненный в области правого подреберья, печень выступает из-под края реберной дуги на 2 см, селезенка – на 1,5. Физиологические отправления в норме. Периферических отеков нет.
По данным эластометрии печени в 2017 г. выявлено наличие фиброза печени (стадия F2, 8,9 кПа по Metavir), при УЗИ брюшной полости выявлены диффузные изменения паренхимы печени, гепатоспленомегалия. УЗИ щитовидной железы и гормоны, ЭКГ, рентгенография легких в пределах нормы.
В связи с наличием противопоказаний к интерферонотерапии, а также наличием ХБП, являющейся противопоказанием к назначению софосбувира, пациентке была назначена ПВТ ХГС «глекапревир+пибрентасвир» в течение 8 недель. Пациентка прошла курс лечения полностью, которое перенесла удовлетворительно с хорошей динамикой гематологических показателей (табл. 2).
Клинический пример 2
Больная К. 37 лет наблюдается в центре с 2005 г. с диагнозом «ВИЧ-инфекция, 4А-стадия, ремиссия на фоне АРВТ. Хроническая цитомегаловирусная инфекция, ремиссия. Хроническая герпетическая инфекция, ремиссия. Вторичная тромбоцитопения. Хронический гепатит С, 3-й генотип, фаза репликации вируса высокой активности, c выраженным фиброзом печени (F4 по METAVIR), обострение. Хронический паренхиматозный панкреатит, ремиссия. ХБП-4. Хронический гломерулонефрит».
При первичном обращении у пациентки был выявлен уровень СD4+Т-лимфоцитов 52 кл/мкл (9%).
С 2014 г. назначена АРВТ по схеме ламивудин+абакавир+эфавиренз, замененная на ламивудин+тенофовир+дарунавир+ритонавир в связи с развитием побочных эффектов.
В 2015 г. при обследовании был выявлен ХГС 3-го генотипа с высокой вирусной нагрузкой 8,4 млн МЕ/мл. ПВТ не проводилась.
В 2016 г. установлен диагноз «хроническая болезнь почек 4-й ст., хронический гломерулонефрит».
Данные объективного осмотра на момент назначения ПВТ: состояние удовлетворительное, кожа чистая, бледная. Лимфоузлы не увеличены, безболезненные. Зев умеренно гиперемирован, на слизистой оболочке полости рта налетов нет. В легких дыхание везикулярное, тоны сердца ясные, ритм правильный. Живот мягкий, умеренно болезненный в области правого подреберья, печень выступает из-под края реберной дуги на 3 см, селезенка – на 1,5. Физиологические отправления в норме. Периферических отеков нет.
По данным эластометрии печени в 2017 г. выявлено наличие фиброза печени (стадия F4, 22,8 кПа по Metavir), при УЗИ брюшной полости имеют место диффузные изменения паренхимы печени, гепатоспленомегалия. УЗИ щитовидной железы и гормоны, ЭКГ, рентгенография легких в пределах нормы.
Учитывая ухудшение самочувствия пациентки, наличие выраженного фиброза печени, гепатоспленомегалии, отсутствие динамики уровня СD4+-лимфоцитов 273 кл/мкл, в отсутствие репликативной активности ВИЧ HIV RNA НПО, настроенность пациентки на лечение ХГС по жизненным показаниям назначена ПВТ ХГС «глекапревир+пибрентасвир» в течение 12 недель.
Произведена смена АРВТ на время лечения ВГС на ламивудин, тенофовир, ралтегравир.
Пациентка полностью прошла курс лечения с достижением УВО, перенесла его хорошо с положительной динамикой гематологических показателей (табл. 3).
Заключение
Схема ПВТ «глекапревир+пибрентасвир» продемонстрировала УВО в отношении 100% больных ХГС и ХБП. Отмечена хорошая переносимость и положительная клинико-лабораторная динамика в процессе лечения и в период последующего наблюдения (12 недель после завершения ПВТ).
По нашему мнению, скрининг маркеров поражения почек следует проводить каждому пациенту с ХГС. Выявление не только признаков явной нефропатии, но и изолированных изменений, таких как микроальбуминурия, снижение СКФ, эпизоды повышения артериального давления, могут быть ранними признаками ассоциированного с ВГС поражения почек и требуют соответствующих дальнейших диагностических и лечебных мероприятий.
В заключение следует отметить, что ассоциированное с ХГС поражение почек достаточно распространено, может иметь различные механизмы развития и многообразные клинико-морфологические проявления.
Очевидно, что осведомленность врачей инфекционистов и нефрологов в этой области медицинских знаний, их более тесное взаимодействие могут существенно улучшать качество и своевременность диагностики и лечения таких пациентов, а следовательно, и отдаленный прогноз.

