Введение
У больных ХБП гиперфосфатемия возникает в результате накопления фосфора в организме за счет потребления фосфатов с пищей и уменьшения их выделения с мочой. Было показано, что гиперфосфатемия – независимый прогностический фактор смертности [1–3]. Целевой уровень фосфора в сыворотке крови должен быть в диапазоне 3,5–5,5 мг/дл для минимизации рисков заболеваемости и смертности [4]. Однако добиться такого уровня фосфора в крови только соблюдением диеты или адекватным диализом не представляется возможным в силу объективных причин. С учетом вышеизложенного трудно переоценить значение применения фосфатсвязывающих препаратов (ФСП) для контроля за уровнем фосфора у больных ХБП. При этом выбор оптимального препарата для коррекции гиперфосфатемии у больных на диализе сегодня – одна из наиболее важных клинических задач. По разным оценкам, до 74% пациентов с терминальной почечной недостаточностью (ТПН) не соблюдают рекомендаций врача (не комплаентны) по приему ФСП [5, 6]. Проблемы с соблюдением режима лечения в первую очередь связаны с приемом большого количества таблеток и с неблагоприятными побочными эффектами.
С учетом низкой эффективности большинства известных ФСП применение нового препарата оксигидроксида железа, не содержащего кальция, и с высокой эффективностью связывания фосфора представляется перспективным.
Целью настоящего проспективного рандомизированного активно контролируемого исследования было оценить эффективность и безопасность нового железосодержащего фосфатсвязывающего препарата – оксигидроксида железа (Вельфоро® 500) и севеламера карбоната для пациентов с гиперфосфатемией, находящихся на лечении программным гемодиализом (ГД).
Материалы и методы исследования
Исследование было проспективным рандомизированным активно контролируемым, проведено в 2019 г. на базе 12-го отделения ГД ГКБ им. С.П. Боткина Москвы. В исследование включены стабильные пациенты (n=50) с ХБП 5, получавшие лечение программным ГД в течение не менее 12 недель до начала исследования и соответствовавшие критериям включения и исключения.
Критерии включения: возраст 18 лет и старше независимо от пола, стандартный режим ГД –3 раза в неделю, диализный Kt/V более 1,2 за процедуру, отсутствие изменения дозы ФСП и других препаратов для коррекции МКН (активаторов рецептора витамина Д, кальцимиметиков) в течение не менее 4 недель до начала периода отмывания; при скрининговом визите уровни P до процедуры ГД должны были быть ≥3,5 мг/дл (1,3 ммоль/л) и <8,0 мг/дл (2,6 ммоль/л), а в течение периода отмывания ≥5,5 мг/дл (1,8 ммоль/л); уровни сывороточного ферритина <1000 мкг/л и насыщения трансферрина (НТЖ) <50%.
Критерии исключения: скорректированный уровень сывороточного Ca менее 1,88 или более 2,75 ммоль/л; уровень интактного ПТГ более 800 пмоль/л; паратиреоидэктомия в анамнезе не менее чем за 6 месяцев до начала исследования; активное/состоявшееся желудочно-кишечное кровотечение или воспалительные заболевания кишечника; стабильно высокие ежемесячные уровни сывороточного Р>10,0 мг/дл (3,2 ммоль/л) на протяжении 3 месяцев до скрининга; поливалентная лекарственная аллергия или непереносимость компонентов лекарств; злокачественные новообразования в анамнезе в течение предшествовавших 5 лет; непереносимость препаратов железа; гемохроматоз в анамнезе или любые другие нарушения накопления железа.
Дизайн исследования: исследование включало скрининг, периоды отмывания (4 недели), титрации доз (8 недель) и лечения (8 недель). Перед началом исследования проводилась рандомизация пациентов с помощью интерактивной системы случайных чисел. Пациенты были распределены для лечения оксигидроксидом железа или севеламером карбонатом в соотношении 1:1. Оба исследуемых препарата назначались перорально 3 раза в день непосредственно во время приема пищи. Начальные дозы оксигидроксида железа были 1 т (2500 мг/500 мг Fe)х3 раза, севеламера карбоната 1 т (800 мг)х3 раза в сутки.
В дальнейшем дозы препаратов титровали каждые 4 недели, исходя из показателей Р, для достижения целевых значений р (1,13–1,8 ммоль/л). Использовались следующие критерии для коррекции дозы. Если концентрация P в сыворотке была более 1,94 ммоль/л, дозу оксигидроксида железа увеличивали на 2500, дозу севеламера – на 2400 мг/день; если она находилась в целевом диапазоне 1,13–1,8 ммоль/л, дозы обоих препаратов не менялись и, если уровни Р были менее 1,13 ммоль/л, доза оксигидроксида железа уменьшалась на 2500, а севеламера карбоната – на 800 или 2400 мг/день. Максимально допустимая доза оксигидроксида железа составила 2500 мг ×3 раза в день (7500 мг/день = 3 табл.), доза севеламера карбоната – 2400 мг×3 раза в день (7200 мг/день = 9 табл.). С 8-й по 16-ю неделю дозы препаратов не менялись. Каждая таблетка оксигидроксида железа содержала 500 мг железа. В течение всего периода исследования был запрещен прием одновременно с исследуемыми препаратами других ФСП, препаратов железа, а также препаратов, влияющих на концентрацию фосфора и кальция в сыворотке.
Критерии прекращения исследования: развитие любого нежелательного явления (НЯ), которое затруднило бы продолжение лечения; определение уровней Р сыворотки крови менее 0,97 или более 3,23 ммоль/л в течение нескольких заборов крови подряд; определение скорректированного уровня Ca в сыворотке крови менее 1,88 ммоль/л и концентрации ферритина более 1000 мкг/л. Прием активных метаболитов витамина D и кальцимиметиков был разрешен, если пациенты получали их до начала исследования в течение 4 недель или более, а дозы этих препаратов оставались неизменными на протяжении всего периода исследования. Ни одному пациенту эти препараты (активные метаболиты витамина D или кальцимиметики) в течение всего периода исследования не были назначены de novo. Между группами различий в использовании этих препаратов не было. Диета, согласно опросу пациентов, на протяжении всего исследования изменялась незначительно.
Забор крови всем больным проводился после двухдневного перерыва до процедуры ГД во время каждого из этапов исследования (период отмывания, титрации доз и лечения).
У всех пациентов ежемесячно оценивались уровни Р, Ca, ПТГ, ферритина, насыщения трансферрина, гемоглобина, интактного фактора роста фибробластов-23 (FGF-23), СРБ. Исследование проводилось в соответствии с этическими стандартами, изложенными в Хельсинкской декларации, пересмотренной в 2013 г. Все пациенты дали письменное информированное согласие до их включения в исследование.
Оценка эффективности и безопасности
Первичным результатом эффективности применения фосфатбиндеров была концентрация фосфора в сыворотке крови в конце лечения. Дополнительные оценки включали концентрацию фосфора в сыворотке крови в каждый момент времени, изменение концентрации фосфора в сыворотке от исходного уровня до конца лечения и показатели достижения целевых концентраций фосфора в сыворотке как 1,13–1,78 ммоль/л. Целевые концентрации фосфора в сыворотке ≥1,13 и ≤1,78 ммоль/л были основаны на целевом диапазоне рекомендаций Ассоциации нефрологов России.
Вторичными результатами эффективности лечения фосфабиндерами были показатели концентрации кальция в сыворотке, концентрации интактного-PTH сыворотки, FGF-23, обмена железа (ферритин, НТЖ), гемоглобина, С-реактивного белка.
Безопасность и переносимость: оценивалась по числу НЯ, СНЯ и отказов больных от продолжения лечения препаратами. Что касается безопасности, НЯ не включали изменения цвета фекалий и языка, вызванные железом, содержащимся в Вельфоро. Число пациентов с событиями и частота событий были рассчитаны в каждой группе.
Статистический анализ: сравнения между группами проводились с использованием критерия Фишера. Уровень значимости был установлен на уровне 5%. Для оценки разницы в полученных данных исследований использовались непараметрические методы, компьютерное ПО SPSS 18.0.
Результаты
В исследование были включены 50 пациентов. После рандомизации в обе группы лечения: оксигидроксида железа (Вельфоро) и севеламера карбоната – включены по 25 пациентов. Из-за развития неблагоприятных явлений (НЯ) из группы оксигидроксида железа выбыли 6 (24%) пациентов, из группы севеламера карбоната – 5 (20%). В общей сложности завершили исследование 19 (75%) и 20 (80%) пациентов групп оксигидроксида железа и севеламера соответственно. Базовые характеристики пациентов приведены в табл. 1. Статистически значимых различий между двумя группами не было.
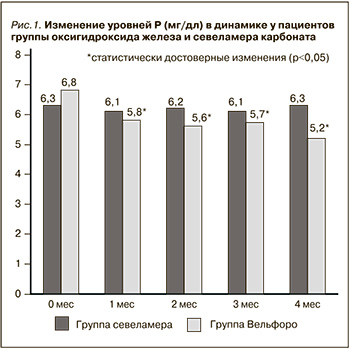
Влияние терапии препаратами оксигидроксида железа (Вельфоро) и севеламера карбоната на сывороточные уровни Р представлено на рис. 1.
Применение оксигидроксида железа сопровождалось достоверным снижением уровней фосфора с 6,8±1,5 до 5,27±0,99 мг/дл (р<0,01), в то время как использование севеламера карбоната не оказало существенного влияния на этот показатель. Изменения лабораторных параметров, отразивших минерально-костные нарушения у пациентов исследуемых групп, представлены в табл. 2.
Достоверных изменений уровней сывороточного Са, иПТГ и FGF-23 отмечено не было.
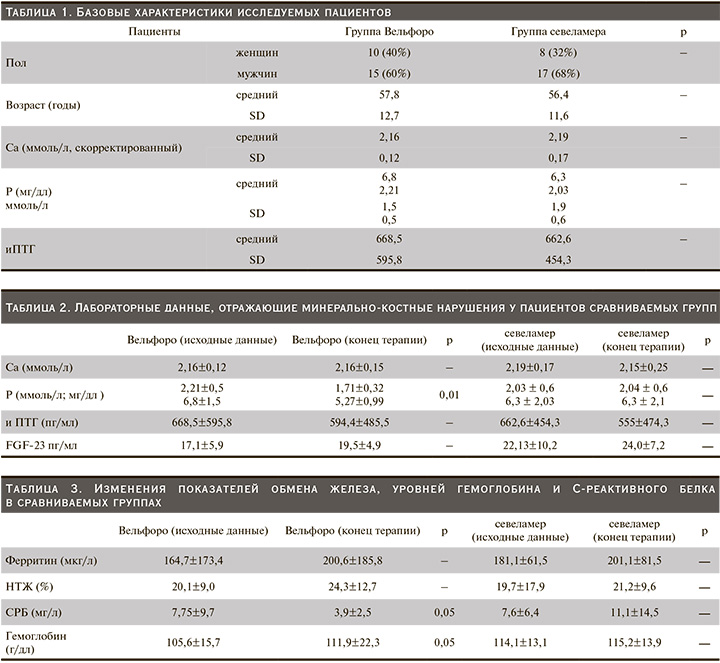
Анализ полученных данных, приведенный в табл. 3, свидетельствует о значимом снижении уровней СРБ – почти на 50% – и достоверном увеличении уровней гемоглобина в группе оксигидроксида железа без существенных изменений показателей обмена железа. Применение севеламера карбоната не оказало существенного влияния ни на один из этих показателей.
 Количество таблеток, которые больные принимали ежедневно в период лечения в группах оксигидроксида железа и севеламера карбоната, составило 2,0±1,5 и 6,1±3,2 табл./сут. соответственно. Среднее суточное количество таблеток, принимаемых пациентами групп сравнения к концу лечения, показано на рис. 2.
Количество таблеток, которые больные принимали ежедневно в период лечения в группах оксигидроксида железа и севеламера карбоната, составило 2,0±1,5 и 6,1±3,2 табл./сут. соответственно. Среднее суточное количество таблеток, принимаемых пациентами групп сравнения к концу лечения, показано на рис. 2.
Безопасность и переносимость
Существенного различия в частоте возникновения нежелательных явлений, связанных с приемом исследуемых препаратов, не наблюдалось. Наиболее частыми нежелательными явлениями во время приема оксигидроксида железа и севеламера карбоната были тошнота (12 и 4%), диарея (20 и 4%), запоры (0 и 20%), дискомфорт в животе (0 и 4%), боли в животе (0 и 4%) соответственно. Большинство случаев диареи были легкими, преходящими и развивались только в начале лечения. Всего из исследования по разным причинам выбыли 11 пациентов: 6 – из группы оксигидроксида железа и 5 – из группы севеламера карбоната.
Обсуждение
В ходе нашего исследования показано, что концентрация фосфора в сыворотке крови при применении Вельфоро достоверно снизилась и в конце лечения находилась в диапазоне контрольных целевых значений (от 1,13 до 1,78 ммоль /л). В то же время прием севеламера карбоната не привел к значимому изменению уровня фосфора в крови. В более ранних работах со схожим дизайном также была показана эффективность Вельфоро [7–9]. В исследовании Jürgen Floege et al. [8] применялись более высокие дозы севеламера, что, возможно, и привело к снижению уровня Р к концу исследования в отличие от наших данных. Объяснением слабого влияния севеламера на уровень Р в крови является, вероятно, его слабая фосфатсвязывающая способность (Р, мг/г ФСП) по сравнению с другими фосфатбиндерами и особенно в отношении оксигидроксида железа (21 против 260 мг/г соответственно) [10]. Подтверждением служат и данные недавнего систематического обзора исследований Кокрановской базы [11], где отмечена гетерогенность результатов в снижении уровня фосфора в крови на фоне приема севеламера и слабая его эффективность в отобранных для анализа исследованиях.
По нашим данным, среднесуточное потребление таблеток было заметно меньше в группе Вельфоро – 2,0±1,5 против 6,1±3,2 табл./сут. в группе севеламера. В исследовании Chiu et al. также показано, что прием большого количества таблеток, по различным причинам (в среднем 19 табл./сут.), негативно влияет на качество жизни пациентов на гемодиализе, а также ухудшает их приверженность к лечению [12]. В то время как уменьшение их количества приводит к лучшему контролю Р в крови [13].
Ряд изучаемых лабораторных параметров (ПТГ, Са, ферритин, НТЖ, FGF-23) достоверно не менялся при применении исследуемых препаратов, что подтверждается и другими авторами [8, 11, 14, 15]. В то же время в нескольких работах показано снижение FGF-23 и иПТГ на фоне этих ФСП [16–19]. Объяснить разницу в полученных результатах может степень влияния фосфатбиндеров на уровень Р. Так, там, где уровень фосфора не менялся или степень его снижения была выражена незначительно, не отмечено и снижения уровней иПТГ и FGF-23.
Повышение уровня гемоглобина на фоне приема оксигидроксида железа, по данным нашего исследования, позволяет обсуждать благоприятный эффект этого препарата в отношении коррекции анемии. Схожее влияние препарата на увеличение уровня Hb также было показано в исследовании Hisato Shima et al. [20]. Эти результаты объяснялись частичной кишечной абсорбцией железа, физиологического металла, содержащегося в оксигидроксиде железа [21]. Однако в нашем наблюдении достоверных изменений уровней ферритина и НТЖ отмечено не было. С этой точки зрения заслуживает большего внимания эффект оксигидроксида железа в отношении уменьшения уровня воспаления (достоверное уменьшение уровня СРБ), что, вероятно, и повлияло на увеличение гемоглобина в этой группе пациентов. Влияние ФСП на уровень воспаления был также показан ранее и в других работах [22–26].
С точки зрения безопасности, хотя диарея и часто наблюдалась в группе Вельфоро, большинство случаев были легкими и преходящими. Только четверо пациентов прекратили участие в исследовании из-за диареи; другие пациенты продолжили прием препарата. После прекращения приема лекарства диарея прекратилась у всех больных. Многие пациенты с ХБП, получавшие процедуры ГД, страдают от запоров. Прием севеламера сопровождался усугублением этого симптома у 4 больных, вынужденных прекратить лечение.
К ограничениям исследования можно отнести открытый дизайн, стандартизацию лабораторных анализов (пациенты посещали диализный центр в разное время суток), потребление фосфатов в рационе пациентов.
Заключение
Применение оксигидроксида железа (Вельфоро® 500) в группе больных ХБП, получавших лечение программным ГД, и гиперфосфатемией было эффективным и безопасным. При этом среднесуточное потребление таблеток было значительно ниже в этой группе Вельфоро, чем в группе севеламера. Напротив, лечение севеламером не продемонстрировало существенного влияния на уровень фосфатов крови, что, возможно, связано с недостаточной дозой препарата и требует дальнейших исследований. Кроме того, применение оксигидроксида железа было ассоциировано с увеличением уровня гемоглобина, а также со снижением уровня воспаления, что может благоприятно влиять на улучшение клинических исходов.



