Введение
Признаки острого почечного повреждения (ОПП) выявляют у каждого пятого госпитализированного пациента, а по данным P.M. Palevsky et al. (2008), у больных, находящихся в критическом состоянии, частота развития ОПП достигает 30%, если же пациенту показано проведение заместительной почечной терапии (ЗПТ), смертность превышает 50% [1]. Чрезвычайно многообразен спектр заболеваний и состояний, осложнением которых является ОПП: сепсис, обширные оперативные вмешательства, низкий сердечный выброс и гиповолемия, травма, применение нефротоксичных препаратов, повышение внутрибрюшного давления, рабдомиолиз др. [2].
Термин «белково-энергетическая недостаточность» (БЭН) вне зависимости от причин ее развития используют в отношении больных ОПП и хроническим заболеванием почек для описания состояния дефицита запасов протеина и энергии (снижение тощей и жировой масс тела) [3]. БЭН встречается у 42% больных ОПП и ассоциирована с ухудшением прогноза заболевания – увеличением смертности и длительности госпитализации, кровотечениями, дыхательной недостаточностью, инфекционными осложнениями [4].
К сожалению, изучению особенностей применения искусственного питания (ИП) больными ОПП посвящено крайне мало фундаментальных исследований и большинство из них проведено на рубеже XX–XXI вв., когда лечение пациентов в критических состояниях и практика применения ИП (предпочтение отдавали парентеральному питанию) существенно отличались от современных подходов. По этой причине современные рекомендации основаны на экспертном мнении специалистов в области применения ИП больными ОПП в критических состояниях.
Особенности метаболизма
Двумя ключевыми механизмами, ответственными за изменения метаболизма углеводов у больных ОПП, служат инсулинорезистеность и усиление глюконеогенеза в печени за счет аминокислот, высвобождаемых на фоне катаболизма протеинов [5, 6].
Своевременная коррекция гипергликемии у больных, находящихся в критическом состоянии, может оказывать протективный эффект на функцию почек [7]. В соответствии с рекомендациями авторов KDIGO 2012 г., пациентам в критическом состоянии предлагается использовать инсулинотерапию для поддержания целевого уровня глюкозы 110–149 мг/дл (6,1–8,3 ммоль/л) в плазме крови [8]. При решении вопроса о применении инсулинотерапии необходимо учитывать, что на основании ряда исследований независимыми факторами риска неблагоприятного исхода для больных в критическом состоянии являются не только гипергликемия, но и гипогликемия, а также амплитуда колебаний уровня глюкозы крови, причем у больных сахарным диабетом пороговые значения отличаются от таковых у пациентов с догоспитальной эугликемией [9, 10]. В связи с этим авторы рекомендаций Surviving Sepsis Campaign 2016 г. с целью снижения риска развития гипогликемии предлагают в качестве целевого уровня с учетом больных сахарным диабетом использовать концентрацию глюкозы ≤180 мг/дл (10 ммоль/л) и применять протоколы для контроля гликемии у больных сепсисом [11].
Замедление клиренса липидов и развитие гипертриглицеридемии при ОПП связывают со снижением активности липазы на ≈50% (периферической липопротеиновой и печеночной триглицеридной) [12]. Поэтому особенно при парентеральном использовании липидных эмульсий необходимо ежедневно контролировать триглицериды плазмы крови и при уровне >400 мг/дл (≈5,3 ммоль/л) останавливать инфузию до нормализации показателей. Кроме того, скорость инфузии подбирают таким образом, чтобы суточная дозировка липидов поступала в течение 18–24 часов [13].
Гиперкатаболизм у больных ОПП развивается вследствие сочетания неспецифических, связанных с острым течением основного заболевания и его осложнений, и специфических изменений (нарушения почечной функции и проведения ЗПТ). Доминирующим механизмом ускоренного распада протеина, как уже отмечено, служит стимуляция в печени глюконеогенеза из аминокислот. Инсулинорезистентность, в результате которой снижается синтез и увеличивается деградация протеина, также играет важную роль в нарушениях белкового обмена при ОПП. Среди дополнительных факторов выделяют метаболический ацидоз, секрецию катаболических гормонов (катехоламины, глюкагон, глюкокортикостероиды), гиперпаратиреоидизм, высвобождение медиаторов воспаления, тип и интенсивность проводимой ЗПТ и др. [14].
Попытка уменьшить выраженность катаболизма протеина с помощью увеличения уровня поступающей с парентеральным питанием энергии с 30 до 40 ккал/кг/сут не увенчалась успехом: баланс азота в обеих группах больных оставался сопоставимым, но гипералиментация пациентов (группа 40 ккал/кг/сут) приводила к развитию гипергликемии, гипертриглицеридемии и гипергидратации [15]. Более того, с точки зрения современных представлений мала вероятность самой возможности скорректировать нарушения биоэнергетического статуса больных в критическом состоянии только за счет увеличения поступления субстратов питания [16]. Одним из перспективных методов, позволяющим активизировать процессы анаболизма, снизить выраженность мышечной атрофии и улучшить исходы заболевания, считается ранняя реабилитация (мобилизация) больных с помощью нейромышечной стимуляции, велоэргометрии, ходьбы и др. [17, 18].
Методы оценки статуса питания
Международным сообществом по изучению метаболизма и применения лечебного питания при заболеваниях почек в качестве критериев БЭН предложено использовать четыре категории параметров, характеризующих снижение ряда биохимических показателей, мышечной и общей массы тела, потребления больным пищи (табл. 1) [3]. По данным авторов, снижение одного из показателей из трех представленных категорий служит основанием для диагностики у больного недостаточности.

Важно учитывать, что у больных ОПП, находящихся в критическом состоянии в связи с острым течением заболевания, развитием системного воспалительного ответа и нарушениями водного баланса, применение данных критериев ассоциировано с неадекватной оценкой статуса питания (низкой чувствительностью и специфичностью) [13].
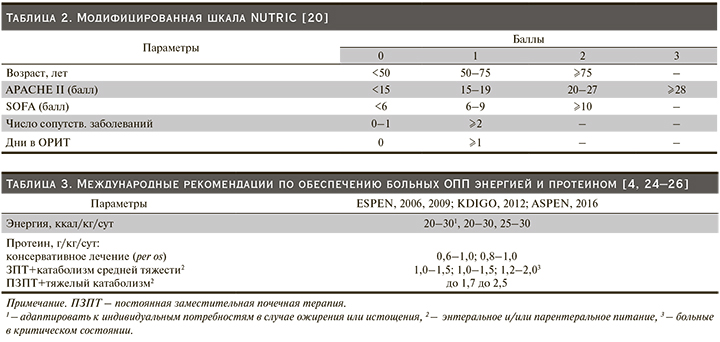
Для выявления риска развития недостаточности питания предложен ряд многофакторных шкал, например NRS (2002) или шкала NUTRIC (2011) для больных в критическом состоянии, однако исследований, характеризующих их применение непосредственно пациентами с ОПП, не проводилось [19, 20]. В табл. 2 представлен модифицированный вариант шкалы NUTRIC (исключен один показатель – концентрация в крови интерлейкина-6). Авторы считают, что при уровне 0–4 балла у больного низкий риск развития недостаточности питания, а если у пациента ≥5 баллов, возникают и необходимость в длительном проведении искусственной вентиляции легких (ИВЛ), и высокий риск развития неблагоприятных исходов, а «агрессивное» применение ИП позволяет существенно улучшить результаты лечения.
Динамическое наблюдение за показателями состава тела служит важным дополнительным параметром как для характеристики статуса питания и баланса жидкостей, так и для эффективности проводимого лечения пациента. Для определения компонентов состава тела (тощая, жировая, мышечная массы, жидкостные секторы) у больных в критическом состоянии активно изучают эффективность использования компьютерной или магнитно-резонансной томографии, ультразвукового исследования, биоимпедансного анализа [21–23].
Стандартный подход к назначению ИП
Изолированное ОПП не оказывает значимого влияния на потребности пациента в энергии, поэтому в отсутствие возможности использовать непрямую калориметрию (НК), назначая ИП, ориентируются на тяжесть течения основного заболевания, его осложнения и статус больного на догоспитальном этапе [13].
В зависимости от тяжести катаболизма, обусловленного течением основного заболевания и необходимости в проведении ЗПТ, предложено разделять пациентов с ОПП на три группы [14]. В первую включают больных без признаков катаболизма (ОПП чаще всего развивается в результате лекарственной или контрастиндуцированной нефропатии). Статус питания пациентов данной группы в подавляющем большинстве случаев остается в пределах нормальных значений – больные могут самостоятельно принимать пищу перорально, и прогноз заболевания благоприятный. Вторая группа представлена пациентами средней степени тяжести состояния и катаболизма в связи с развитием инфекционных осложнений, перитонита, применением хирургических вмешательств и потенциальной необходимостью в использовании ЗПТ. Данной группе обычно требуется энтеральное и/или парентеральное питание. Третью и наиболее многочисленную группу составляют больные, у которых ОПП развивается как компонент полиорганной недостаточности, например, на фоне тяжелой травмы, ожоговой болезни или сепсиса. В этой группе применяют комплексное лечение, в состав которого входят зондовое энтеральное и/или парентеральное питание, различные виды ЗПТ, ИВЛ, вазопрессорная поддержка и др.
Согласно рекомендациям ESPEN (2006, 2009), KDIGO (2012), ASPEN (2016), уровень общего количества калорий, необходимых больному при любой стадии ОПП, в среднем составляет 20–30 ккал/кг/сут (табл. 3) [4, 24–26]. Рекомендации ESPEN (2006, 2009), KDIGO (2012) и ASPEN (2016) в отношении необходимого уровня потребления протеина различаются. Согласно ESPEN (2006, 2009) и KDIGO (2012), для пациентов без признаков катаболизма достаточный уровень потребления протеина составляет 0,6–1,2 г/кг/сут, при средней тяжести состояния/катаболизма и/или необходимости в проведении ЗПТ – 1,0–1,5, в случае развития гиперкатаболизма и применения продленных методов ЗПТ – до 1,7 г/кг/сут. Авторы рекомендаций ASPEN (2016) предлагают увеличить верхний уровень потребления протеина до 2 г/кг/сут, для больных, требующих частого проведения диализов, до 2,5 г/кг/сут. Однако следует отметить, что, по результатам исследования R. Bellomo et al. (2002), у больных ОПП, находящихся в критическом состоянии, применение высоких доз протеина (>2,5 г/кг/сут) хотя и сопровождается некоторым улучшением белкового баланса, но не влияет на исходы заболевания и требует более агрессивного проведения ЗПТ [27]. Более того, анализируя данные, полученные в исследовании RENAL (2008), авторы выявили, что в реальной клинической практике средний уровень потребления протеина пациентами с ОПП составляет 0,5 г/кг/сут (>1 г/кг/сут только у 11% больных), энергии – 11 ккал/кг/сут. Смертность была сопоставимой среди больных с уровнями суточного потребления протеина и энергии выше (43%) и ниже среднего (46%) [28, 29]. Таким образом, в настоящее время в связи с отсутствием достаточной доказательной базы нет оснований рекомендовать высокие дозы протеина широкой популяции больных ОПП в критическом состоянии.
Современный подход к назначению ИП
Составляя план питания для больного ОПП, находящегося в критическом состоянии, необходимо учитывать, что с точки зрения метаболических нарушений данная группа пациентов крайне неоднородна. Изменения метаболизма непосредственно связаны с основным заболеванием и его осложнениями, острым нарушением функции почек и влиянием, оказываемым ЗПТ [14]. Если сравнить рекомендованные международными обществами значения потребления энергии и протеина с данными, опубликованными в обзоре G. Kreymann et al. (расход энергии и потери протеина пациентами с различными заболеваниями), можно заключить: приблизительно у 30% пациентов применение рекомендаций будет ассоциировано с риском избыточного или недостаточного назначения ИП [30]. Кроме того, потребности в ИП могут различаться не только между отдельными больными, но и у одного и того же пациента с течением времени в зависимости от фазы заболевания [31].
В отличие от НК попытки использовать для оценки расхода энергии расчетные формулы, в т.ч. включающие такие параметры, как пол, возраст и минутная вентиляция, неадекватно отражают изменения метаболизма, характерные для пациентов с острыми и хроническими заболеваниями [32, 33]. Еще одной проблемой в отделениях интенсивной терапии остается необходимость определения потребности больного в энергии и протеине, исходя из расчетных формул или рекомендованных значений, основанных на массе тела, что может приводить к избыточному или недостаточному назначению ИП, принимая во внимание существенные различия в значениях идеальной, рекомендованной или фактической (частое развитие гипергидратации, ожирение) масс тела, сухом весе пациента [34].
Таким образом, несмотря на ряд ограничений (обусловленных необходимостью измерения объема поглощенного пациентом кислорода и выделенного углекислого газа), использование НК считается «золотым» стандартом определения расхода энергии больными в критическом состоянии. Клинические ситуации, требующие аккуратной интерпретации данных, полученных при НК, включают ажитацию больного и неадекватное обезболивание/седацию, сброс воздуха в контуре ИВЛ>10% минутного объема, нестабильную температуру и рН крови, насыщение воздушной смеси кислородом >60%, проведение заместительной почечной или печеночной терапии (переохлаждение, бикарбонатный буферный раствор и т.д.), экстракорпоральной мембранной оксигенации (необходимы дополнительные датчики потока в контуре аппарата) [31, 35].
Расчет баланса азота для больных в критическом состоянии помогает определить выраженность катаболизма и целевые значения потребления протеина. Баланс азота подразумевает соответствие между потреблением и потерями азота с учетом около 0,5 г/сут азота (рост волос, ногтей, кожного покрова, выделение пота, заборы крови и т.д.) [36]. Поскольку оценка общих потерь азота в связи с трудоемкостью и затратами до настоящего времени в рутинной клинической практике невозможна, принято использовать для расчетов основной продукт деградации белка и аминокислот – азот мочевины мочи (у стабильных больных обычно составляет 80–90% общего азота мочи) [37]. Среди нестабильных пациентов с печеночной недостаточностью, ОПП, тяжелым сепсисом потери азота за счет других компонентов (аммоний, мочевая кислота) могут вносить более существенный вклад, а ошибки расчета достигать 12 г азота в сутки [38]. Необходимо также учитывать потери азота по дренажам, при обширных раневых поверхностях (больные с ожоговой травмой), кишечных свищах. Развитие олигурии или анурии, снижение клиренса креатинина ˂50 мл/мин/1,78 м2 ограничивают использование стандартного метода оценки потерь азота с мочой [39]. В связи с этим уровень катаболизма протеина у больных, требующих проведения ЗПТ, оценивают по более сложной формуле с учетом азота мочевины крови, мочевины мочи и диализата (ультрафильтрата, например) [40]:
Потери азота (г/сут)=азот мочевины мочи (г/сут)+азот мочевины диализата (г/сут)+изменение уровня азота мочевины крови (г/сут).
Изменение уровня азота мочевины крови (г/сут)=(АМК2-АМК1, г/л/сут)×МТ1 (кг)×(0,6 л/кг)+(МТ2- МТ1, кг/сут)×АМК2 (г/л)×(1,0 л/кг).
АМК – азот мочевины крови (г/л), МТ – масса тела (кг), 1 – начальное (постдиализное), 2 – конечное (предиализное) значение.
Таким образом, несмотря на ограничения и основываясь на клиническом опыте и здравом смысле, оптимальный подход к определению потребностей больного в питании должен быть индивидуальным. В зависимости от возможностей стационара он включает оценку текущего статуса питания (анамнез, антропометрия, многофакторные шкалы), изменения компонентов состава тела в течение госпитализации (тощая, жировая, мышечная массы, баланс жидкости), использование НК, расчет баланса азота, определение уровня триглицеридов, протеинов, витаминов и микроэлементов плазмы крови.
Энтеральное и парентеральное питание
В соответствии с современными принципами применения ИП пациентами, находящимися в критическом состоянии, подавляющему большинству больных ИП начинают с раннего энтерального питания (ЭП) [41]. Под ранним понимают ЭП в течение 48 часов пребывания больного в отделении интенсивной терапии. Внимательно следят за симптомами плохой переносимости питания, начатого с небольшой скоростью: 10–20 мл/ч. При хорошей переносимости скорость инфузии постепенно (обычно в течение 2–3 дней) увеличивают до целевых значений. В зависимости от тяжести симптомов (гастростаз, дискомфорт, тошнота, болевой синдром, увеличение внутрибрюшного давления, подозрение на нарушение мезентериального кровоснабжения и др.) решают вопрос о применении прокинетиков (метоклопрамид, эритромицин), интестинального введения растворов и продолжения ЭП или его временного прекращения.
Поскольку в настоящее время нет данных об оптимальных значениях уровней энергии и протеина, необходимых в ранней фазе острого критического состояния, задачи за короткий промежуток времени достичь целевых значений перед медицинским персоналом не стоит. Более того, избыточное поступление питания в ранней фазе может ухудшить результаты лечения за счет снижения активности аутофагии и гипералиментации (увеличение частоты инфекционных осложнений, длительности ИВЛ, развитие гипергидратации и др.), и по данным ряда исследований, гипокалорийное (<70% от целевого уровня) раннее ЭП по меньшей мере безопасно при некоторых критических состояниях и компенсированном статусе питания больного до госпитализации [42–45].
Показания к проведению парентерального питания среди пациентов с ОПП существенно не отличаются от рекомендаций для больных в критических состояниях без признаков ОПП (кишечная непроходимость, ишемия, синдром короткой кишки, синдром интраабдоминальной гипертензии и др.), но ряд особенностей важно учитывать [46]. При решении вопроса о необходимости использования дополнительного парентерального питания (недостаточный объем ЭП) оптимальным подходом к предотвращению осложнений, связанных с гипералиментацией больных, остается применение НК [47]. Доступные в нашей стране коммерческие продукты для зондового ЭП часто не позволяют достигать целевых значений уровня потребления протеина (>1,2 г/кг/сут) без гипералиментации, особенно больными, требующими проведения продленных методов ЗПТ. Данным пациентам может потребоваться дополнительная парентеральная инфузия растворов аминокислот. С целью снижения риска развития гипертриглицеридемии, как уже было отмечено: длительность инфузии суточной дозы липидных эмульсий должна составлять 18–24 часа.
Специфические аспекты больных, требующих проведения ЗПТ
Молекулярная масса свободных аминокислот, микроэлементов, водорастворимых витаминов, глюкозы, фосфата ниже, чем коэффициент просеивания современных мембран для проведения гемодиафильтрации.
Потери глюкозы при постоянных методах ЗПТ могут достигать 30–60 г/сут и при необходимости требуют увеличения концентрации глюкозы в растворах замещающей жидкости [48, 49].
Нормализованный уровень катаболизма протеина у больных, требующих проведения ЗПТ, в среднем составляет 1,5 г/кг/сут (1,4–1,8 г/кг/сут), общая потеря аминокислот за сутки соответствует 10–15 г (0,2 г аминокислот на 1 литр ультрафильтрата), в связи с чем для компенсации потерь увеличивают количество назначаемого протеина на 0,2 г/кг/сут [13].
Гипертриглицеридемия – нечастое осложнение у больных ОПП, но за счет увеличения вязкости крови, активации проконвертина и других факторов может сопровождаться ранним развитием тромбозов гемофильтра [13, 50].
В соответствии с рекомендациями ESPEN (2006, 2009) ЭП в объеме 1500–2000 мл/сут обеспечивает пациента стандартными дозами, а применение парентерального питания требует дополнительного назначения витаминов и микроэлементов [24, 25]. Потребности больных ОПП в микроэлементах и витаминах подробно не изучены, и, по всей видимости, изменения их уровней обусловлены основным заболеванием, интенсивностью проведения ЗПТ [51]. Остается открытым вопрос клинической значимости и дозировок дополнительного (к стандартному суточному уровню) назначения отдельных витаминов и микроэлементов, особенно в отсутствие возможности лабораторной оценки их дефицита. По данным M.M. Berger et al. ([52], самая высокая концентрация в эффлюенте выявлена для селена, концентрация хрома и меди также снижена в крови больных ОПП, но, т.к. цинк содержится в замещающей жидкости, особенно в бикарбонатных растворах, его общий баланс остается положительным. Водорастворимые витамины в отличие от жирорастворимых удаляются из плазмы крови при проведении ЗПТ, в связи с чем рекомендуется их дополнительное применение, в особенности тиамина, фолиевой кислоты и витамина С [53]. Однако избыточное применение витамина С больными ОПП может стать причиной развития вторичного оксалоза [54]. В экспериментальных исследованиях отмечено, что в связи с ОПП уровень ретинола в крови может быть повышен, но в клинических исследованиях выявлено снижение (возможно, в связи с течением основного заболевания) концентрации таких жирорастворимых витаминов, как А, D, E, но не витамина К [55, 56].
Одним из методов улучшения статуса питания пациентов с ОПП, находящихся в критическом состоянии, является применение ежедневных гибридных или продолжительных методов ЗПТ, что позволяет и контролировать уровень водно-электролитного баланса, и адекватно проводить ИП, объем которого в среднем за сутки у взрослых больных составляет 1500–2000 мл [57].
Еще одной важной особенностью ИП является необходимость учитывать при расчете уровня потребления больным энергии не только лекарственных препаратов, например жировых эмульсий (пропофол), но и скрытых калорий в растворах, применяемых при проведении ЗПТ. Цитрат, используемый в качестве антикоагулянта, служит источником энергии для клеток. При расчетах выявлено, что по завершении стандартной процедуры ЗПТ около 300–500 ммоль цитрата остается в кровотоке, что соответствует дополнительному поступлению 100–200 ккал/сут. Если же в качестве антикоагулянта используют ACD-A (состоит из цитрата, лимонной кислоты и 2,5%-ного раствора декстрозы), а буферный раствор содержит лактат, то суммарное количество дополнительных калорий, поступающих в кровоток, может достигать 1200 за сутки [58, 59].
В отличие от пациентов с хронической почечной недостаточностью для больных ОПП, находящихся в отделениях интенсивной терапии, характерна гипофосфатемия, частота развития которой увеличивается при использовании ЗПТ [60]. Тяжелая гипофосфатемия ассоциирована с развитием дыхательной недостаточности, слабостью дыхательных мышц, а также дисфункцией миокарда и энцефалопатией [61, 62]. Одним из вариантов снижения частоты развития гипофосфатемии, особенно в связи с проблемой доступности парентеральных форм фосфата, считается использование замещающих растворов, содержащих фосфат [63].
С увеличением дозы фильтрации повышается риск развития гипотермия [64]. Потери энергии при снижении температуры тела могут составлять около 800 ккал/сут, и если эффект гипотермии не используется специально для коррекции, например, злокачественной гипертермии или гиперметаболизма-гиперкатаболизма с высокой температурой, увеличение температуры венозного контура до 43ºС позволяет снизить потери тепла на 72% [65].
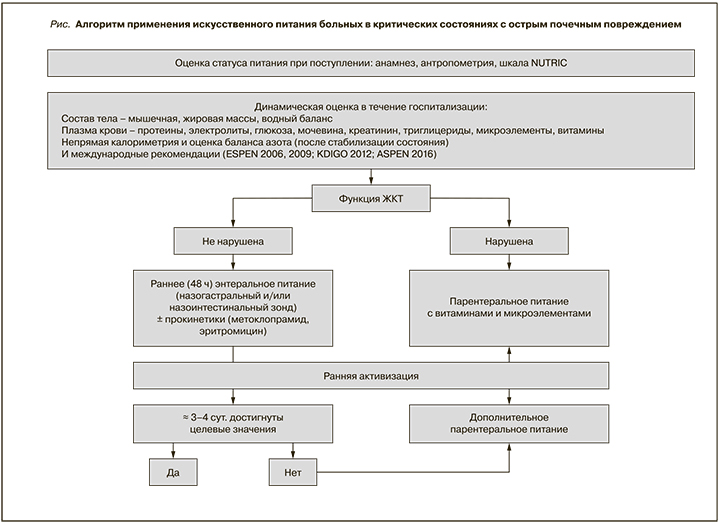
Заключение
Высокая частота развития БЭН при ОПП, особенно среди пациентов с полиорганной недостаточностью, требует адекватного назначения макро- и микронутриентов. Гетерогенность популяции больных ОПП в критическом состоянии определяет необходимость дифференцированного подхода к оценке статуса питания и назначению ИП с использованием НК, расчета баланса азота, учета метаболических изменений, вызванных развитием ОПП, и потерь, связанных с применением ЗПТ. Таким образом, с точки зрения современного понимания проблемы применения ИП в перспективе оптимален индивидуальный подход с использованием многофакторного анализа показателей статуса питания больного в критическом состоянии. В соответствии с предложенным подходом на рисунке представлен базовый алгоритм принятия решений при назначении ИП больным ОПП в критическом состоянии.

