Хроническая HCV-инфекция достаточно распространена среди пациентов с хронической болезнью почек (ХБП), особенно при заместительной почечной терапии программным гемодиализом (ПГД) [1]. Распространенность HCV-инфекции у пациентов с тХПН высока среди больных, получающих лечение гемодиализом, и колеблется от 7,3 до 16,8% [2]. В России антитела к HCV выявляют у 11,3% больных, получающих заместительную почечную терапию [3]. В то же время отмечается снижение распространенности HCV-инфекции, что связано с введением рутинного серологического скрининга, активным применением эритропоэтина, осуществлением процедур по инфекционному контролю, а также с внедрением этиотропного лечения диализных пациентов.
HCV-инфекция у диализных больных характеризуется медленным прогрессированием, бессимптомным течением, низкой активностью аминотрансфераз и низкой вирусной нагрузкой [4–6]. Тем не менее HCV-инфекция у диализных пациентов ассоциируется с повышенным риском смерти от заболевания печени и сердечно-сосудистых осложнений [7, 8]. В то же время трансплантация почки в значительной степени изменяет течение вирусного гепатита С. Применение иммуносупрессивных препаратов в посттрансплантационном периоде приводит к значительному увеличению вирусной нагрузки и способствует быстрому формированию цирроза печени (ЦП). Увеличивается частота внепеченочных осложнений, а также HCV-индуцированного гломерулонефрита, что сокращает сроки функционирования донорского органа [9, 10]. HCV-инфекция не служит противопоказанием к трансплантации почки, т.к. она позволяет увеличивать отдаленную выживаемость больных [11]. Учитывая неблагоприятные последствия HCV-инфекции, эксперты KDIGO рекомендуют противовирусную терапию (ПВТ) HCV-инфицированным больным тХПН, находящимся в листе ожидания трансплантации почки [2].
До недавнего времени стандартом лечения HCV-инфекции у диализных пациентов считалась терапия интерфероном и рибавирином, эффективность которой не превышала 60% [12]. Частота нежелательных явлений и прекращения ПВТ диализными пациентами была выше, чем у пациентов с нормальной функцией почек. ПВТ больных тХПН на основе интерферона ассоциировалась с развитием тяжелой трудноконтролируемой анемии, которая требовала назначения высоких доз эритропоэтина и/или переливания эритроцитарной массы, что значительно удорожало ПВТ на основе интерферона. Лечение интерфероном и рибавирином было возможным только до трансплантации почки, т.к. после операции интерферон значительно увеличивает риск отторжения почечного трансплантата. Если же в диализный период удалось достичь эрадикации вируса, то после трансплантации почки рецидива HCV-инфекции не наблюдалось, несмотря на комбинированную иммуносупрессивную терапию [13].
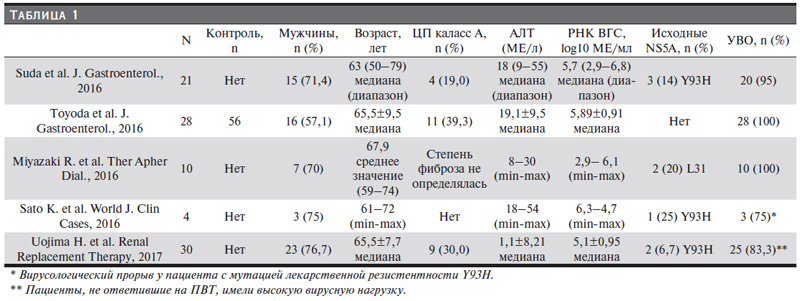
В связи с этим необходимы безопасные и эффективные противовирусные препараты, которые можно было бы применять для лечения HCV-инфекции у диализных пациентов. Одной из первых безинтерфероновых и безрибавириновых схем ПВТ хронического гепатита С (ХГС) является комбинация препаратов прямого противовирусного действия ингибитора NS5A даклатасвира и ингибитора NS3-протеазы асунапревира [14]. Даклатасвир и асунаправир метаболизируются печенью и элиминируются с желчью, что позволяет применять их в отношении больных тХПН без коррекции дозировки [15, 16]. Данная схема имеет удобный режим применения: 2 раза в сутки независимо от приема пищи и процедур гемодиализа [17, 18]. К преимуществам можно отнести отсутствие рибавирина, а к относительным недостаткам, как у всех препаратов прямого действия, – это межлекарственные взаимодействия и потенциал лекарственной резистентности. Результаты первых клинических исследований, в которых участвовали в общей сложности 93 диализных пациента с хроническим гепатитом С, вызванным вирусом 1b-генотипа, показали высокую эффективность и хорошую переносимость 24-недельной комбинированной терапии даклатасвиром и асунапревиром (табл.1) [19–23]. Частота устойчивого вирусологического ответа (УВО) через 12 недель после завершения противовирусной терапии достигла 83,3–100,0%, в т.ч. у больных компенсированным ЦП. Лечение хорошо переносилось и было безопасным. Среди нежелательных явлений можно выделить повышение трансаминаз, что расценивалось как гепатотоксичность препаратов прямого действия, а из особенностей – более быстрый клиренс РНКHCV у пациентов с тХПН, чем у больных без нарушения функции почек [20].
В 2015 г. комбинированная терапия асунапревиром и даклатасвиром была зарегистрирована в Российской Федерации для лечения хронического гепатита С 1в-генотипа. Однако до настоящего времени отсутствует опыт применения данной схемы пациентами с тХПН.
Целью исследования было изучение эффективности и безопасность асунапревира 200 мг в сутки и даклатасвира 60 мг в сутки в течение 24 недель для пациентов с хронической HCV-инфекцией генотип 1в и тХПН, получавших лечение программным гемодиализом в условиях реальной клинической практики.
Материал и методы
С декабря 2015 по ноябрь 2016 г. были обследованы 18 пациентов с 1в-генотипом хронической HCV-инфекции. Критерии включения: наличие продвинутой стадии фиброза печени или ЦП класса А по Чайлд–Пью и/или присутствие в листе ожидания трансплантации почки [24]. Критерии исключения: ЦП класса В и С по Чайлд–Пью, прием препаратов, запрещенных к совместному применению с асунапревиром и даклаласвиром; наличие мутаций лекарственной резистентности. Всем пациентам для определения степени фиброза печени проведена эластометрия [25], повторное определение генотипа вируса гепатита С и вирусной нагрузки. Десять пациентов имели минимальную степень фиброза, у 5 диагностирована продвинутая стадия фиброза печени или ЦП. При повторном обследовании у одной больной РНК вируса гепатита С не определен, у одного – выявлен подтип 1а. Восемь пациентов находились в листе ожидания трансплантации почки. Одному из пациентов комбинацию даклатасвир и асунапревир не назначили в силу наличия взаимодействия с другими принимаемыми препаратами [17, 26].
Были отобраны семь пациентов с 1в-генотипом. Всем им проведено секвенирование вируса гепатита С для уточнения наличия первичных мутаций лекарственной резистентности (МР) к препаратам прямого действия (ФБУН «Центральный НИИ эпидемиологии» Роспотребнадзора). У одного больного определена клинически значимая мутация к асунапревиру (S122G), из исследования этот пациент был исключен. Ни у одного больного не было выявлено клинически значимых МР к даклатасвиру. Перед ПВТ проведены общий анализ крови (гемоглобин, тромбоциты), биохимический анализ крови (АСТ, АЛТ, общий билирубин, альбумин), коагулограмма (ПИ), УЗИ брюшной полости, ЭГДС, анализ сопутствующей терапии. Шесть пациентов получали даклатасвир (60 мг 1 раз в день) и асунапревир (100 мг 2 раза в день) в фиксированных дозах амбулаторно. Лечение продолжали в течение 24 недель. Клинико-демографические показатели больных представлены в табл. 2.
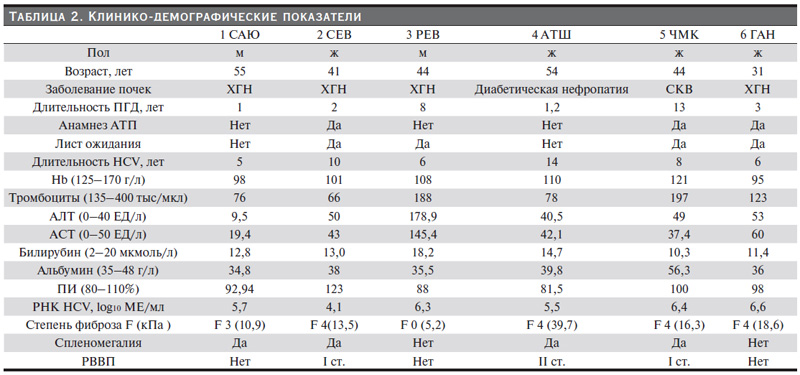
Причиной тХПН у четырех пациентов стал хронический гломерулонефрит, у одного – диабетическая нефропатия, у одного – системная красная волчанка. Три пациента имели в анамнезе аллотрансплантацию почки. Четыре пациента находились в листе ожидания. ЦП диагностирован у четырех пациентов (с помощью эластометрии среднее значение составило более 12,5 кПа, что соответствовало ЦП). У одного больного была выраженная стадия фиброза печени (F3). У четырех пациентов выявлены признаки портальной гипертензии в виде спленомегалии, у троих из них – варикозно расшренные вены пищевода. У больных циррозом синтетичная функция печени была в норме. У четырех выявлена тромбоцитопения. Уровень трансаминаз в большинстве случаев не превышал 1,5 нормы, кроме больного 3 РЕВ. Он страдал минимальной степенью фиброза. Однако он планировал родственную трансплантацию почки, в связи с чем был включен в исследование.
Для оценки эффективности определяли сывороточную концентрация HCV-РНК. Контроль безопасности осуществлен путем определения лабораторных показателей, включая АЛТ, АСТ, тромбоциты. Обследование проведено на 2-й, 4, 8, 12, 24-й неделе непосредственно перед процедурой гемодиализа, чтобы исключить ее влияние на лабораторные показатели. Пациентов наблюдали еще в течение 12 недель после завершения ПВТ с последующей оценкой устойчивого вирусологического ответа, динамики фиброза печени, уровней АЛТ, АСТ и тромбоцитов.
Результаты
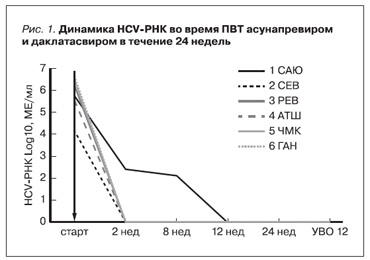 У 5 пациентов авиремия наблюдалась через 2 недели от начала ПВТ и сохранялась на всем протяжении лечения. У пациента 1 САЮ отмечено медленное снижение клиренса HCV-РНК, вирус гепатита С перестал определяться только к 12-й неделе ПВТ. Все шесть больных достигли непосредственного вирусологического ответа (авиремия через 24 недели ПВТ). У всех пациентов сформировался устойчивый вирусологический ответ, произошла эрадикация (рис. 1).
У 5 пациентов авиремия наблюдалась через 2 недели от начала ПВТ и сохранялась на всем протяжении лечения. У пациента 1 САЮ отмечено медленное снижение клиренса HCV-РНК, вирус гепатита С перестал определяться только к 12-й неделе ПВТ. Все шесть больных достигли непосредственного вирусологического ответа (авиремия через 24 недели ПВТ). У всех пациентов сформировался устойчивый вирусологический ответ, произошла эрадикация (рис. 1).
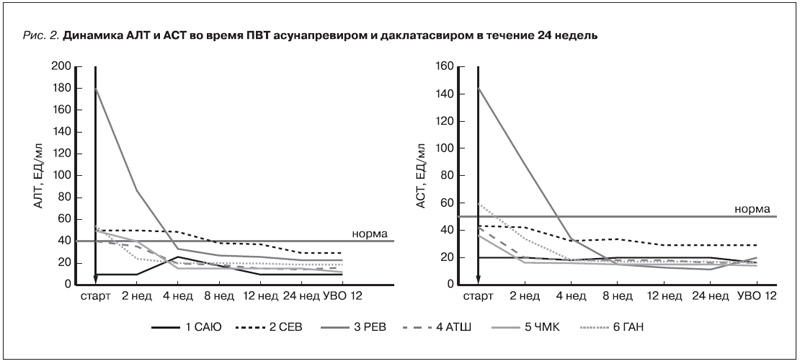
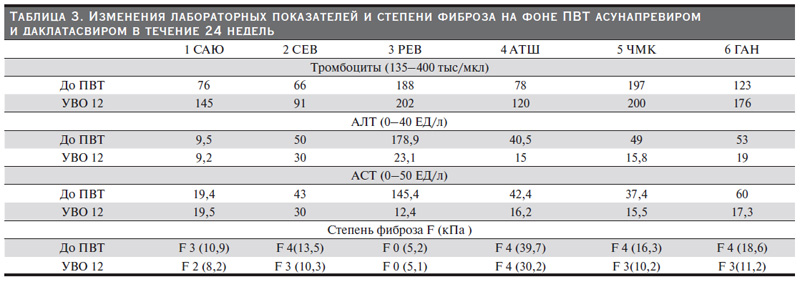
На фоне ПВТ у 5 пациентов с биохимической активностью отмечено снижение уровней АЛТ и АСТ (рис. 2). Ни у одного пациента гепатотоксичности препаратов прямого противовирусного действия зафиксировано не было. После достижения УВО 12 у четырех пациентов с продвинутой стадией фиброза печени или ЦП отмечено уменьшение степени фиброза печени и у пяти – уменьшение жесткости печени по данным эластометрии, у троих больных тромбоцитопенией – нормализация тромбоцитов (табл. 3).
Серьезных нежелательных явлений не отмечено, случаев смерти не было.
Обсуждение
Мы изучили эффективность и безопасность даклатасвира и асунапревира для диализных пациентов с HCV-инфекцией. Комбинированная терапия была высокоэффективной для диализных пациентов с генотипом HCV-1в, в т.ч. для больных ЦП. УВО 12 был достигнут 100% больных (6/6). По сравнению с интерферонотерапией даклатасвир и асунапревир хорошо переносились диализными пациентами [27]. Лечение даклатасвиром и асунапревиром привело к улучшению сывороточных концентраций АЛТ, АЛТ и уменьшению степени фиброза печени. Хотя число пациентов было небольшим, полученные данные свидетельствуют о том, что комбинированная терапия даклатасвиром и асунапревиром эффективна и безопасна для пациентов с тХПН, инфицированных яHCV 1в-генотипа.
Лечение ХГС имеет важное значение для диализных больных, т.к. HCV-инфекция значительно ухудшает прогноз у пациентов, находящихся на гемодиализе, по сравнению с таковым у недиализных пациентов [28, 29]. В первую очередь лечение следует назначать кандидатам на трансплантацию почки, т.к. исходы ее ухудшаются у HCV-позитивных пациентов [30]. До последнего времени единственным методом лечения HCVинфекции у диализных больных была терапия пегилированным интерфероном [2]. Частота УВО при этом была невысокой, а ПВТ часто вызывала нежелательные явления [27, 31–33]. Даклатасвир – это первый ингибитор неструктурного белка 5A (NS5A), многофункционального протеина, необходимого для репликации вируса гепатита C. Он подавляет два этапа жизненного цикла вируса – репликацию вирусной РНК и сборку вирионов. Дакластвир обладает высокой активностью в отношении всех генотипов HCV. Асунапревир – это мощный ингибитор NS3/4 протеазы, активен в отношении только 1в-генотипа. Асунапревир нарушает процессинг полипротеина вируса гепатита C для получения зрелых вирусных белков, требуемых для репликации вируса. Даклатасвир метаболизируется в печени под действием цитохрома-P450 (CYP) 3A4 [18], а асунaпревир – под действием CYP3A и выводится преимущественно с калом (84%) [17]. Следовательно, оба препарата можно применять даже больным, находящимся на гемодиализе.
В клинических исследованиях пациентов с ПГД УВО на фоне ПВТ ауснапревиром и даклатасвиром может достигать 100%, в т.ч. у пациентов с ЦП [19–21]. В исследовании Suda и соавт. из 21 пациента с тХПН 4 (19 %) имели ЦП и все они достигли УВО [19]. Другое более крупное исследование контрольной группы, в котором учувствовали 28 человек с ПГД, из них ЦП наблюдался у 11 (39,3%), продемонстрировало 100%-ную эрадикацию HCV [20]. Наше исследование также показало высокую эффективность данной схемы, в т.ч. при выраженной стадии фиброза печени/циррозе. Стоить отметить, что у четырех наших пациентов имели место признаки портальной гипертензии в виде спленомегалии, у троих – расширение варикозных вен пищевода; у троих течение HCV-инфекции осложнилось тромбоцитопений.
Одним из ограничений препаратов прямого действия является наличие МР, которые могут снижать эффективность терапии, что было продемонстрировано на пациентах с нормальной функцией почек [34–36]. В популяции пациентов с ХБП данные о распространенности и влиянии МР на УВО противоречивы. У одной больной с наличием МР к даклатасвиру наблюдалась терапевтическая неудача [22]. По данным других исследований, МР к даклатасвиру не препятствуют эрадикации вируса гепатита С [19, 23]. В нашем исследовании наличие МР как к асунапревиру так и к даклатасвиру служило критерием исключения. Из семи обследованных больных тХПН ни у кого не было выявлено исходных мутаций в регионе NS5A (даклатасвир), у одного пациента присутствовала к ингибиторам протеазы HCV (асунапревир). Несмотря на то что эффект мутаций к ингибиторам протеазы вируса гепатита С может быть небольшим [37], из данного исследования пациент был исключен.
У пациентов, получающих ПГД с ХГС, уровни сывороточных трансамназ находятся на более низких значениях. Показано, что средняя концентрация АЛТ у диализных больных HCVинфекцией составляет 22,8 ЕД/л, у диализных пациентов без HCV – 16,1 ЕД/л [5]. В нашем исследовании до назначения ПВТ только у одного больного уровень трансаминаз был в норме. У четырех больных ЦП отмечена незначительная биохимическая активность не более 1,5 норм. Вероятно, эти пациенты имели выраженную активность печеночного процесса. Высокую биохимическую активность мы наблюдали у одного больного 3 РЕВ с минимальной степенью фиброза печени. Одним из побочных эффектов даклатасвира и асунапревира является увеличение активности АЛТ, которое иногда может достигать 3–4-й степеней и требовать прекращения терапии [34]. В связи с этим мы уделили особое внимание изменениям концентрации АЛТ и АСТ. Однако мы не зафиксировали ни одного случая повышения этих ферментов. Нормализации «печеночных ферментов» произошла к 4-й неделе, а после окончания лечения концентрация АЛТ и АСТ значительно снизилась и все больные достигли полного биохимического ответа.
В нашем исследовании тромбоцитопения наблюдалась у 4 из 6 пациентов. При ХВГ С тромбоцитопения может быть обусловлена снижением синтетической функции печени при сформированном циррозе, гиперспленизмом в рамках портальной гипертензии и спленомегалии, а также аутоиммунными механизмами как системное проявление HCV. В эпоху интерферонов тромбоцитопения относилась к противопоказаниям к проведению ПВТ и была одним из распространенных нежелательных явлений интерферонов [37]. Кроме того, тромбоцитопения служила предиктором терапевтической неудачи [39]. В настоящее время низкое количество тромбоцитов не ограничивает использования препаратов прямого действия. В нашем исследовании тромбоцитопения не относилась к противопоказаниям ПВТ и выявлена у 4 из 5 больных выраженным фиброзом печени. После достижения УВО у всех четырех больных тромбоцитопенией мы отметили увеличение их количества.
Эрадикация вируса гепатита С ассоциируется с уменьшением степени фиброза печени, что было продемонстрировано в многочисленных клинических исследованиях пациентов с нормальной функцией почек [40, 41]. По нашим данным, у 4 больных выраженным фиброзом после достижения УВО отмечено уменьшение фиброза и у 5 – уменьшение жесткости печени, определенной с помощью эластометрии. Нам представляется это крайне важным фактом. Четверо больных находятся в листе ожидания трансплантации почки, трое уже имели в анамнезе пересадку почки. Кроме того, у всех эти больных наблюдалась тяжелая степень фиброза/цирроз. Применение иммуносупрессивных схем в посттрансплантационном периоде может приводить к обострению ВГС и быстрому прогрессированию заболевания печени, что может значительно ухудшить жизненный прогноз. Неопределенный срок в листе ожидания трансплантации у больных ПГД и ЦП рано или поздно приведет в декомпенсации заболевания печени и, возможно, будет стоять вопрос о сочетанной трансплантации печени и почки. Достижение УВО после успешной ПВТ, возможно, приведет к уменьшению степени фиброза и регрессу заболевания печени.
Основным недостатком нашего исследования были ограниченное число пациентов и незначительный период наблюдения. Таким образом, мы показали, что комбинированная терапия даклатасвиром и асунапревиром высокоэффективна и хорошо переносится диализными пациентами, в т.ч. при ЦП. Наши данные свидетельствуют о том, что комбинация двух препаратов может безопасно применяться в лечении диализных больных, инфицированных 1в-генотипом ХГС. С учетом малочисленности наблюдений необходимы более крупные исследования.



