Введение
Инфекционные осложнения у пациентов с мочеточниковыми катетерами-стентами – актуальная проблема, остро стоящая перед специалистами урологических клиник. За последние годы плотность инцидентности катетер-ассоциированных инфекций мочевых путей (КАИМП) составляет от 4,1 до 8,8 на 1000 катетеро-дней и является одним из наиболее распространенных типов внутрибольничной инфекции [1, 2]. Установкой стента заканчиваются до 60% вмешательств по устранению конкрементов в мочеточнике и до 80% вмешательств по элиминации конкрементов из почки [3, 4]. К основным факторам риска развития катетер-ассоциированных инфекций мочевых путей (ИМП) относят женский пол, возраст, наличие сопутствующих заболеваний, длительность катетеризации, нарушение правил инфекционной безопасности и др. [5, 6].
Известно, что установка любых дренажей в мочевую систему приводит к их микробной контаминации. Такая инфекция называется катетер-ассоциированной и имеет особенности в виде образования микробных ассоциаций, обладающих повышенной резистентностью к антибактериальным препаратам [7]. Наиболее часто выявляемыми бактериями при колонизации стентов являются Е. coli, K. pneumoniae, P. aeruginosa, S. aureus, Е. faecium в монокультуре или в составе ассоциаций с наличием ряда генетически детерминированных факторов адгезии, таких как уреазопозитивность, сукцинатдегидрогеназа и т.п. [8, 9]. При этом оценка чувствительности микроорганизмов демонстрирует высокую резистентность изолятов к основным антибактериальным препаратам, применяемым в урологической практике. Назначение антибиотиков безусловно оправданно в качестве основного средства периоперационной профилактики или лечения инфекционных осложнений при эндоскопических операциях с установкой в верхние мочевыводящие пути внутренних дренажей – мочеточниковых стентов. Однако их применение при КАИМП с бессимптомной бактериурией не рекомендуется, т.к. многие энтеробактерии становятся полирезистентными, продуцируют бета-лактамазы расширенного спектра, также растет резистентность к фторхинолонам, цефалоспоринам широкого спектра действия и к карбапенемам и из-за угрозы развития побочных эффектов [10, 11]. Уменьшению степени микробной контаминации дренажей может способствовать использование растительных уросептиков и диуретиков, обладающих противовоспалительными, антиадгезивными и антимикробными свойствами [12]. Растительные препараты хорошо переносятся больными, возможно их длительное использование в непрерывном режиме без побочных эффектов. Одним из эффективных растительных средств является отечественный лекарственный препарат ПростаНорм®, в состав которого входят официнальные экстракты смеси растений: корней солодки, травы зверобоя, травы золотарника канадского, корневищ эхинацеи пурпурной. ПростаНорм® обладает выраженными противовоспалительным, анальгезирующим и диуретическим свойствами, что позволяет рассматривать его в качестве перспективного антибактериального средства для профилактики и лечения КАИМП [13, 14].
Цель исследования: оценка клинической эффективности растительного лекарственного препарата ПростаНорм® у пациентов с мочекаменной болезнью (МКБ) и установленными катетерами-стентами мочеточника после выполнения рентгенурологических эндоскопических операций.
Материалы и методы
В проспективное исследование были включены 40 пациентов женского пола с МКБ и установленными мочеточниковыми стентами после выполнения рентгенурологических эндоскопических операций (контактной уретеролитотрипсии – КУЛТ) в возрасте от 32 лет до 81 года (54,1±9,7). Все операции были выполнены в отделении урологии ГБУЗ МО МОНИКИ. Исследование одобрено Независимым комитетом по этике при ГБУЗ МО МОНИКИ им. М.Ф. Владимирского (заседание № 5 от 20.10.10.2018).
Критерии включения в исследование: пациенты, ознакомившиеся с информацией по исследованию и подписавшие информированное согласие на участие в нем, женский пол, возраст от 18 до 85 лет, подтвержденный диагноз МКБ, планируемая операция КУЛТ с типом устанавливаемого стента-JJ, мочеточниковый катетер-стент (C.R. Bard, Германия; BIORAD MEDISYS, Индия).
Критерии невключения/исключения: больные острым пиелонефритом/обострением хронического пиелонефрита, необходимость проведения антибиотикотерапии, прием других антибактериальных средств, в т.ч. и растительного происхождения, непереносимость/аллергическая реакция на прием препарата ПростаНорм®.
Всем больным выполнено ультразвуковое исследование почек и верхних мочевыводящих путей c использованием ультразвуковой системы Flex Focus BK Medical (Дания) через сутки после установки стента для контроля корректности его положения и отсутствия дилатации чашечно-лоханочной системы почки. В исследование включены только пациенты с адекватным функционированием стента и его правильным положением в верхних мочевых путях.
Пациенты были разделены на две группы: 20 человек составили основную группу и 20 – контрольную. Пациентам основной группы одновременно с установкой стента назначался ПростаНорм® в виде монотерапии в непрерывном режиме с длительностью приема 1 месяц. Препарат ПростаНорм® был предоставлен компанией АО «ФПК ФармВИЛАР» в двух лекарственных формах: таблетки, покрытые оболочкой, получаемые из экстракта из смеси лекарственного сырья – травы зверобоя, травы золотарника канадского, корней солодки, корневищ с корнями эхинацеи пурпурной в соотношении 1:1:1:1, и жидкий экстракт того же состава. В течение первых 2 недель пациенты принимали по 2 таблетки, покрытые оболочкой, 3 раза в сутки, в последующие 2 недели – жидкий экстракт по 40 капель 3 раза в сутки. Больным контрольной группы не назначали антибиотиков и/или других растительных препаратов.
Исследования выполнялись троекратно: на 1–3-и сутки после установки стента и начала приема ПростаНорм®, через 2 недели (через 1–2 суток после удаления стента) и через 4 недели. Клинический анализ мочи и бактериологический посев выполнялся в те же сроки.
Эффективность лечения оценивали на основе динамики жалоб на учащенное мочеиспускание – поллакиурию, с учетом числа дневных и ночных микций, а также по результатам клинического и бактериологического исследования мочи.
Статистический анализ полученных данных проводили с использованием пакета прикладных программ SPSS Statistics.
21.0. Стандартная обработка выборок включала подсчет значений средних арифметических величин, ошибок средних, а также величины дисперсии и среднего квадратичного отклонения. Сравнение показателей по количественным признакам осуществляли непараметрическим методом с использованием теста согласованных пар Вилкоксона или U-критерия Манна– Уитни. При сравнении двух групп с нормальным характером распределения данных использовали t-тест для независимых группировок. Для всех видов анализа статистически значимыми считали различия при р<0,05.
Результаты и обсуждение
Стентирование верхних мочевыводящих путей с целью поддержания проходимости мочеточника широко используется в урологической практике. Однако наряду с очевидными преимуществами этой хирургической манипуляции (малая травматичность, отсутствие наружного дренажа и др.) специалисты сталкиваются с рядом проблем, связанных с возможной бактериальной колонизацией, функциональными, воспалительными и микроциркуляторными нарушениями в дренируемом сегменте, а также с риском устойчивости к противомикробным препаратам, что приводит к негативным последствиям, требует длительной госпитализации, а также увеличения экономических затрат [8]. Эффективная лечебная тактика может помочь минимизировать подобные осложнения.
В настоящем исследовании внимание уделено особенностям динамики некоторых клинико-лабораторных показателей пациентов, получающих терапию отечественным антимикробным растительным препаратом ПростаНорм®.
После установки стента у обследованных женщин в обеих клинических группах частота микций не различалась и в среднем составляла 9–10 раз в сутки. В основной группе после удаления стента (2 недели от начала исследования) было отмечено снижение частоты микций в среднем на 30% – 6,0±1,8. В контрольной группе на этом этапе исследования достоверных различий в частоте микций не наблюдалось и составляло 10,0±1,9 в сутки. Через месяц после начала приема ПростаНорм® в основной группе частота микций снизилась до 5,0±1,1 в сутки, в контрольной – до 6,0±1,1.
В клиническом анализе мочи оценивали динамику числа лейкоцитов и эритроцитов. Как видно из рис. 1 в период наблюдения снижение лейкоцитурии имело общую тенденцию в обеих группах, по-видимому, связанную с наличием катетера-стента и последующим его удалением.
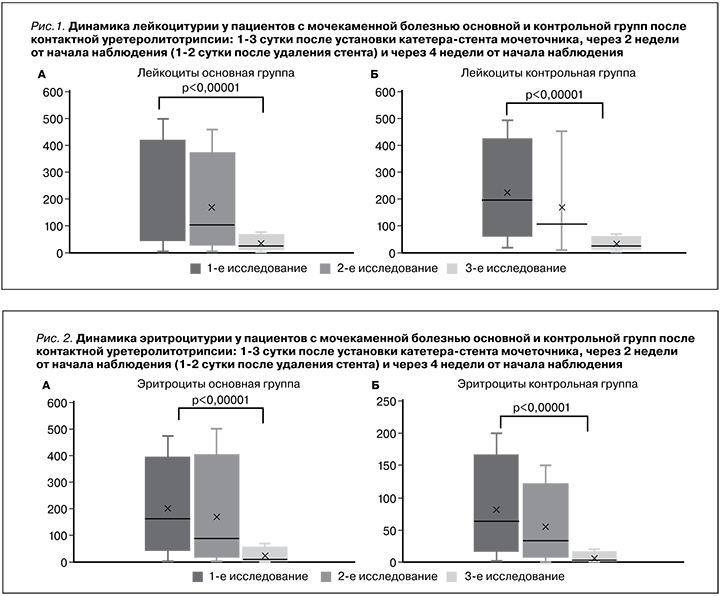
Регресс эритроцитоурии также имел общую тенденцию в обеих группах (рис. 2).
Статистически значимые изменения (p<0,00001) были зарегистрированы только через месяц после начала наблюдения, т.е. через 2 недели после удаления стента. Можно предположить, что число эритроцитов и лейкоцитов в моче имело общую тенденцию к снижению, связанную непосредственно с «механическим» воздействием катетера-стента в мочеточнике и последующим его удалением, а значит, не может быть расценено как критерий эффективности проводимого лечения.
Бактериологическое исследование мочи после установки катетера-стента мочеточника в обеих группах показало отсутствие роста микроорганизмов всего в 5% проб. В 86% проб с наличием роста преобладали грамотрицательные микроорганизмы, среди которых ведущими были E. coli (57%), K. pneumoniaе (29%), P. aeruginosa (14%). В остальных (9%) пробах была представлена грамположительная флора двух видов: Enterococcus spp. (90%) и S. haemolyticus (10%). Дрожжеподобные грибы C. albicans были выделены из 5,5% проб. Микроорганизмы выделялись в концентрации 104 –105 КОЕ/мл.
Наши результаты в основном согласуются с данными других авторов по этиологической расшифровке КАИМП у женщин: в общей структуре возбудителей обычно преимущественно лидируют грамотрицательные микроорганизмы (E. coli, E. agglomerans, P. mirabilis и др.); 5–7% случаев встречаются грибы рода Candida [15–18].
Роль грибковых возбудителей инфекций за последние годы все более возрастает. В большинстве случаев выявление дрожжеподобных грибов Candida в моче (кандидурия) является следствием колонизации мочевых путей по дренажу [19]. При этом в этиологической структуре грибы Candida могут составлять до 27–40% и даже занимать первое место среди всех возбудителей КАИМП у пациентов отделений интенсивной терапии в многопрофильных стационарах [20, 21]. Эти факты свидетельствуют об особом значении нарушения нормальной микрофлоры в развитии кандидоза и кандидурии организма вследствие неадекватной и необоснованной антибактериальной терапии [22, 23].
Через 2 недели от начала наблюдения после удаления мочеточникового стента в основной группе пациентов, получавших ПростаНорм®, доля проб с отсутствием роста составляла 15%. В контрольной группе, напротив, рост микрофлоры наблюдался во всех пробах. Перечень видов микроорганизмов в обеих группах оставался прежним, но концентрация значительно снижалась: в основной группе до 103 КОЕ/мл, а в контрольной – до 104 КОЕ/мл.
Спустя месяц от начала лечения при бактериологическом исследовании отсутствие роста в основной группе отмечено уже в 25% случаев, т.е. у каждого четвертого пациента. В то же время в контрольной группе роста не было всего в 15% проб. В основной группе высевались преимущественно Enterococcus spp. в концентрации менее 103 КОЕ/мл, следующими по частоте встречаемости были E. сoli, составившие 80%, в единичных случаях – P. aeruginosa и K. pneumoniaе. В контрольной группе состав микрофлоры оставался прежним и соответствовал результатам второго исследования, однако концентрация снижалась также до 103 КОЕ/мл.
Приведенные клинико-лабораторные данные свидетельствуют о том, что препарат ПростаНорм® обладает выраженным антибактериальным действием. Это позволяет заключить, что данный отечественный растительный лекарственный препарат может быть рекомендован при длительном лечебном и профилактическом режиме терапии пациентов с катетерассоциированной инфекцией в моче.
Выводы
Многочисленными исследованиями, проведенными в течение последних 20 лет, убедительно доказано, что смесь лекарственных трав, входящих в состав препарата ПростаНорм®, позволяет улучшать микроциркуляторные процессы, нормализовывать мочеотделение, оказывает противовоспалительное, капилляропротекторное, анальгетическое и антимикробное действия. Наличие хорошего терапевтического эффекта при относительной безвредности фитопрепаратов стимулирует поиск наиболее рационального их применения, а также расширения их функционала при лечении различных нозоологий. Наши результаты убедительно продемонстрировали возможность достижения положительного лечебного действия фитопрепарата на пациентов с МКБ и установленными мочеточниковыми стентами на более ранних сроках, что способствует снижению риска развития возможных инфекционных осложнений.
Таким образом, применение растительного лекарственного препарата ПростаНорм® в урологической клинике можно рассматривать в качестве перспективной альтернативной стратегии в решении проблем КАИМП.



