Введение
Результаты EMPAREG-OUTCOME, CANVAS, CREDENCE, DECLARE-TIME служат основанием того, что ингибиторы натрий-глюкозного ко-транспортера 2 типа (иНГЛТ-2) в последнее время рекомендуются для лечения пациентов с сахарным диабетом 2 типа (СД2) и высоким сердечно-сосудистым риском [1]. Пациенты с посттрансплантационным СД (ПТСД) также имеют высокий сердечно-сосудистый риск [2].
ПТСД может возникать после трансплантации почки. У 10–20% реципиентов ПТ без предшествующего анамнеза СД развивается гипергликемия – в основном из-за применения высоких доз иммуносупрессивной терапии [3–5]. Развитие ПТСД связано с повышенным риском сердечно-сосудистых заболеваний и плохим прогнозом для выживаемости пациента [6]. Сердечно-сосудистые осложнения у пациентов с ПТСД служат результатом непрерывного кардиоренального взаимодействия. Поэтому их лечение должно быть комплексным, нацеленным на устранение множества факторов риска. Применение иНГЛT-2 стало предпочтительным методом лечения пациентов с СД2 и высоким сердечно-сосудистым риском [7]. Ингибирование НГЛT-2 улучшает контроль гликемии, может уменьшать индекс массы тела (ИМТ) и эпикардиальную жировую массу, снизжать окислительный стресс и асептическое воспаление, улучшать функцию сосудов. иНГЛТ-2 способствуют снижению гиперурикемии, усилению аутофагии и лизосомальной деградации, повышению уровня эритропоэтина и числа циркулирующих проваскулярных клеток-предшественников [8].
Снижение основных сердечно-сосудистых событий, по-видимому, наиболее выраженно у пациентов с установленными сердечно-сосудистыми заболеваниями. Оно не зависит от гипогликемизирующего эффекта иНГЛТ-2, сердечной недостаточности и почечного риска [9].
Наиболее частыми побочными эффектами, о которых сообщалось в клинических и наблюдательных исследованиях, являются инфекции половых органов.
Дискуссионны вопросы взаимодействия сахароснижающих препаратов с иммуносупрессантами и их влияния на фармакокинетику. Несмотря на возможности современной иммуносупрессии, связанной с применением ингибиторов кальциневрина – ИКН (такролимуса), у реципиентов ПТ обычно наблюдается снижение СКФ менее 70 мл/мин/1,73 м2 из-за наличия единственной функционирующей почки [11]. Ингибирование НГЛT-2 изначально снижает внутриклубочковое давление, но со временем стабилизирует функцию почек [12, 13]. Это потенциально может иметь долгосрочный положительный эффект для пациентов с ПТСД как для выживаемости ПТ, так и для прогноза пациента в целом, в том случае если терапия иНГЛТ-2 будет безопасной для реципиентов ПТ.
Целью исследования было изучение возможности безопасного использования иНГЛТ-2 у реципиентов почечного аллотрансплантата (ПАТ) с ПТСД.
Материал и методы
С ноября 2017 по январь 2019 г. В ГБУЗ «Эндокринологический диспансер» ДЗМ были обследованы 104 реципиента ПАТ. У 47 из них диагноз ПТСД был снят в связи с нормальными уровнями гликированного гемоглобина (HbА1с) и гликемии натощак без сахароснижающей терапии. В исследование были включены 57 реципиентов ПАТ с ранее установленным диагнозом ПТСД, согласно критериям Всемирной организации здравоохранения и Российской ассоциации эндокринологов [14, 15] (уровень глюкозы в плазме натощак >7,0 ммоль/л или 2-часовой уровень глюкозы в плазме >11,1 ммоль/л после глюкозотолерантного теста или HbA1c>6,5%), проживших более года после АТП и наблюдавшихся в Городском нефрологическом центре ДЗМ. Критерии включения: возраст старше 18 лет, трансплантация более 1 года назад, стабильная функция почек (отклонение уровня креатинина сыворотки не более чем на 20% в течение последних 2 месяцев) и стабильная иммуносупрессивная терапия в течение как минимум 3 месяцев до включения. Пациенты с расчетной СКФ (рСКФ) менее 30 мл/ мин/1,73 м2, беременные или кормящие матери в исследовании не участвовали. Все пациенты получили устную и письменную информацию в соответствии с надлежащей клинической практикой. До включения в исследование от 12 пациентов получено письменное информированное согласие на проведение терапии иНГЛТ-2. Остальные 45 пациентов от данного лечения отказались и составили группу контроля. Исследование проведено в строгом соответствии с Хельсинкской декларацией Всемирной медицинской ассоциации (1964) и Этическими принципами медицинских исследований с привлечением человека [16].
План исследования включил 5 визитов (исходный уровень, 4-я, 8-я, 16-я и 24-я недели) в течение которых оценивали анамнез, демографические, антропометрические, лабораторные данные (табл. 1) и сопутствующую лекарственную терапию (табл. 2). Все данные регистрировали в амбулаторных картах. Участников проинформировали о том, что они должны придерживаться своей обычной диеты и физических упражнений в течение периода исследования, а сопутствующая терапия должна оставаться неизменной, если только не было снижения уровня глюкозы, пограничного по гипогликемии для пациентов с СД (≤3,9 ммоль/л).
Соблюдение режима лечения, безопасность оценивали на 4-й, 8-й, 16-й и 24-й неделях. Анализ безопасности включал данные физикального обследования, взятие стандартных образцов крови и мочи, определение минимальных уровней иммуносупрессантов. Минимальные уровни иммуносупрессантов проанализированы в лаборатории Городского нефрологического центра ГКБ № 52 ДЗМ. Образцы крови натощак и утренняя моча для оценки мочевого осадка, экскреции глюкозы, креатинина, белка и альбумина исследовали в клинико-диагностической лаборатории Эндокринологического диспансера ДЗМ.
Конечные точки исследования включили изменение уровня HbA1c, глюкозы в плазме натощак, ИМТ, окружности талии/окружности бедер (ОТ/ОБ), артериального давления, рСКФ, микроальбуминурии (МАУ) и соотношения альбумина к креатинину в утренней моче исходно и на 24-й неделе. Дополнительно оценивалась динамика показателей липидного спектра (холестерин – ХСобщ., липопротеиды низкой плотности – ЛПНП, липопротеиды высокой плотности – ЛПВП, триглицериды), фосфорнокальциевого обмена (кальций общий и кальций ионизированный, фосфор, паратиреоидный гормон), мочевой кислоты, эритропоэза (снижение показателя гематокрита, эритроциты, Hb) и функциональной активности β-клеток (НОМА-β).
СКФ рассчитывали в соответствии с формулой MDRD (Modification of Diet in Renal Disease) [17]: СКФ (мл/мин/ 1,73 м²)=175×(Scr)-1,154×(Возраст)-0,203×(0,742 для женщин).
Для оценки функциональной активности β-клеток поджелудочной железы рассчитывали HOMA-β=20 х инсулин натощак (мкЕД/мл)/(глюкоза натощак (ммоль/л) -3,5 [21].
Динамику указанных выше показателей анализировали в виде ∆ (%) разницы в изменении величины исходного уровня и величины, полученной на 24-й неделе лечения. Сравнению подлежали результаты сопряженности ∆ между первой (опытной – пациенты, принимавшие иНГЛТ-2 в комбинации с другими сахароснижающими препаратами) и второй (контрольной – пациенты на стандартной сахароснижающей терапии) группами. Значения p менее 0,05 считались статистически значимыми. Данные представлены в виде медианы (межквартильный размах – IQR). Все статистические анализы выполнены с использованием программного обеспечения SPSS версии 22.0.
Результаты
Всего в исследовани е быливключены 57 пациентов. Все пациенты завершили исследование: 12 в первой группе и 45 в контрольной. В целом группы были сопоставимыми по исходным данным, которые представлены в табл. 1.
Пациенты опытной группы представлены в основном мужчинами (11/1, 91,7%) против 22/23; 48,9%, в контрольной группе. Медианы ИМТ, ОТ/ОБ, были статистически (численно) выше в опытной группе, а ЛПВП – в контрольной. У двух включенных в исследование пациентов: 1 (8,3%) в опытной и 1 (2,2%) в контрольной группах, была повторная АТП. Функция ПАТ соответствовала хронической болезни почек стадии 2–3 [22]. Все участники получали лечение одним или несколькими сахароснижающими препаратами. В опытной группе в комбинации с иНГЛТ-2 чаще применялись ингибиторы дипептидилпептидазы-4 (иДПП-4): n=3/12 (72,7%) против n=27/45 (35,7%); р=0,027 и метформин: n=8/12 (66,7%) против n=9/45 (20,5%); р=0,002. Различий в иммуносупрессивной и сопутствующей терапии между группами не было (табл. 2).
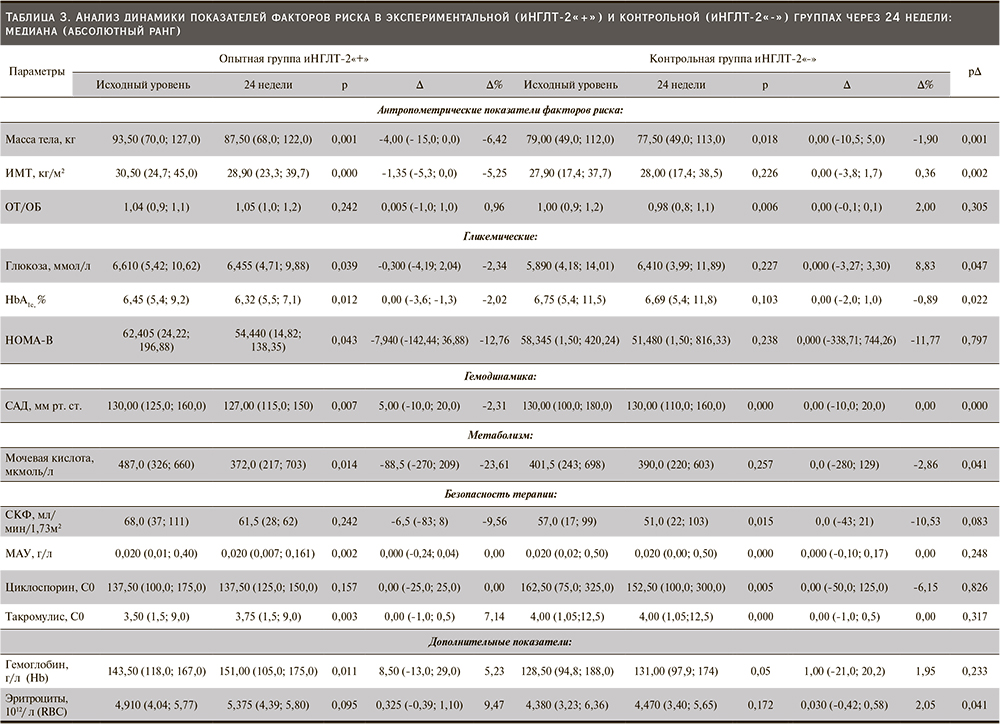
В нашем исследовании обнаружена разница в ∆ контроле НbА1с (р=0,022, рис. 1) и гликемии натощак (р=0,047, рис. 2) между опытной и контрольной группами (табл. 3). Трем пациентам опытной группы была уменьшена сопутствовавшая сахароснижающая терапия. Двум пациентам снизили суточную дозу инсулина с 60 до 50 и с 28 до 12 единиц соответственно. Еще одному пациенту снизили дозу гликлазида с 60 до 30 мг/сут. В то же время в контрольной группе двум пациентам потребовалась интенсификация сахароснижающей терапии: одному увеличена суточная доза инсулина с 24 до 36 единиц, другому назначен глимепирид 2 мг/сут.

Сокращение сахароснижающей терапии в опытной группе и ее усиление в контрольной не сочеталось с изменениями проводимой иммуносупрессивной терапии: медианы минимальных доз ИКН остались прежними, ∆ такролимус С0 р=0,317 (рис. 3), ∆ циклоспорин С0 р=0,826 (рис. 4, табл. 3).
Одному пациенту как опытной, так и контрольной групп изменена иммуносупрессивная терапия с заменой микофенолата мофетила на эверолимус (из-за выявленной базалиомы). Шести пациентам скорректирована доза ИКН: одному контрольной и пяти опытной групп (табл. 4).
Лечение иНГЛТ-2 вызывало достоверное снижение массы тела (р=0,001; рис. 5), ИМТ (р=0,002; рис. 6) по сравнению с группой контроля (табл. 3).
СКФ снижалась в течение первых 8 недель лечения иНГЛТ-2, но впоследствии стабилизировалась. Не было значительных различий в фильтрационной функции почек между группами после 24 недель лечения: ∆ рСКФ -6,5 (-83; 8) против 0,0 (-43; 21); р=0,083 (рис. 7). Медиана соотношения альбумина к креатинину в утренней порции мочи исходно не превышала референсных значений в обеих группах и не изменилась после завершения исследования, ∆ альбумин/креатинин в моче не менялся (табл. 3).
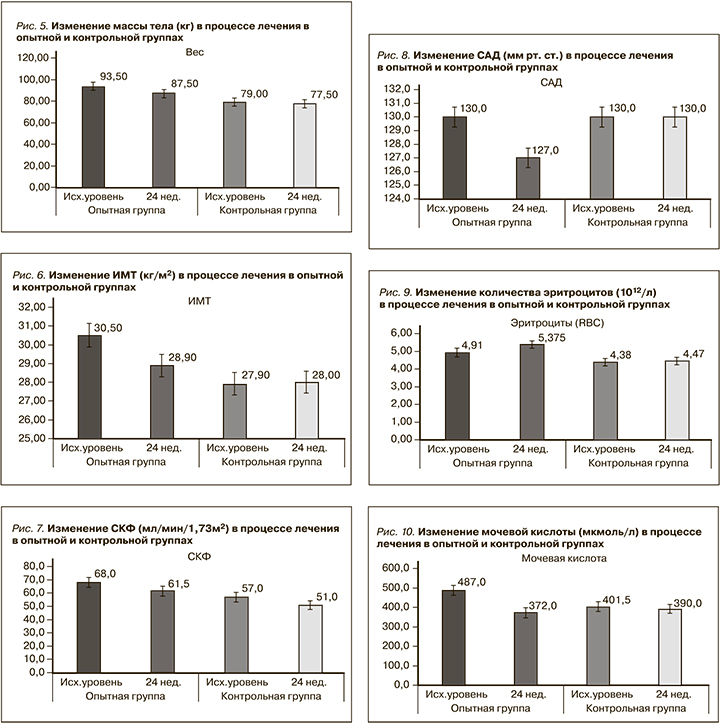
Медиана систолического артериального давления (САД) различалась между опытной и контрольной группами и была достоверно ниже в опытной группе на 24-й неделе терапии иНГЛТ-2: ∆ САД 5,00 (-10,0; 20,0) против 0,00 (-10,0; 20,0); p=0,00 соответственно (рис. 8, табл. 3). Пяти (42%) пациентам, входившим в опытную группу, после 8 недель лечения иНГЛТ-2 снижены дозы принимаемых антигипертензивных препаратов.
У пациентов, получавших иНГЛТ-2, было отмечено увеличение количества эритроцитов: [∆ эритроцитов 0,325 (-0,329; 1,10) vs 0,03 (-0,42; 0,58) P=0,041] (рис. 9). Изменений показателей гемоглобина и гематокрита по сравнению с контрольной группой в нашем исследовании не было р=0,266 и р=0,233 соответственно (табл. 3).
Лечение иНГЛТ-2 привело к значительному медианному снижению уровня мочевой кислоты: -88,5 (-270; 209) против 0,0 (-280; 129); р=0,041 (рис. 10). Показатели липидного обмена между группами после 24 недель лечения не различались: ∆ ХС (р=0,963), ∆ ЛПНП (р=0,903), ∆ триглицеридов (р=0,865), ∆ ЛПВП (р=0,766) (табл. 3).
Определение глюкозурии на фоне применения иНГЛТ-2 рассматривалось как простой тест для оценки приверженности проводимому лечению и было положительным у всех пациентов опытной группы с 4-й по 24-ю неделю исследования. В контрольной группе глюкозурии не выявлено (табл. 4).
Лечение и-НГЛТ-2 переносилось хорошо, без серьезных побочных эффектов. У 1 пациента опытной группы и у 3 контрольной выявлены признаки мочевой инфекции. Этим пациентам назначали терапию уросептиками. Лечение иНГЛТ-2 в то время не прекращалось. Ни у одного из пациентов не было гипогликемии и отторжения ПАТ в течение всего периода исследования (табл. 4).
Заключение
По имеющимся литературным данным, это первое открытое проспективное исследование иНГЛТ-2 у реципиентов ПТ с ПТСД в России. Терапия иНГЛТ-2 оказалась безопасной и эффективной в этой группе пациентов. Лечение иНГЛТ-2 ассоциировалось со снижением уровня НbА1с, гликемии натощак, сопутствовавшим снижением САД, массы тела, ИМТ, уменьшением гиперурикемии и повышением числа эритроцитов после 24 недель лечения. Это может иметь особое значение для пациентов с ПТСД, т.к. изменение данных показателей оказывает положительное влияние на снижение сердечно-сосудистого риска [8, 9, 12, 13, 23].
В связи с этим важно отметить, что сердечно-сосудистые осложнения у пациентов с ПТСД представляют собой результат непрерывного кардиоренального взаимодействия. Лечение иНГЛТ-2 изначально снижает внутриклубочковое давление [13, 23], стабилизирует функцию почек, может устранять множество общих факторов риска хронической болезни почек и сердечно-сосудистых заболеваний, связано с кардио- и ренопротекцией. В нашем исследовании СКФ снижалась в течение первых 8 недель лечения и-НГЛТ-2, но впоследствии стабилизировалась. Первоначальное снижение СКФ при применении и-НГЛТ-2 согласуется с данными других исследований [13, 23]. Дополнительным положительным свойством терапии иНГЛТ-2 является отсутствие гипогликемических явлений за весь период исследования [26].
Инфекции мочевыводящих путей, особенно генитальных, на фоне приема иНГЛТ-2 часто регистрируются как побочные эффекты [27–29]. Можно было бы предположить еще более высокую частоту этих событий у реципиентов ПТ, поскольку их иммунная система подавлена постоянной посттрансплантационной терапией. Однако наше исследование не выявило серьезных побочных эффектов, кроме одного случая мочевой инфекции (без необходимости отмены иНГЛТ-2). В любом случае следует рекомендовать меры предосторожности при использовании иНГЛT-2 в лечении реципиентов ПТ с наличием рецидивирующих инфекций мочевыводящих путей в анамнезе.
Проведенное исследование пациентов, получавших лечение иНГЛТ-2, не выявило каких-либо признаков релевантных фармакокинетических взаимодействий с иммуносупрессантами. Ограничением текущего исследования служит небольшая выборка пациентов. Однако участники исследования представляют популяцию реципиентов ПТ с ПТСД, которым впервые были назначены иНГЛТ-2.
Лечение иНГЛТ-2 хорошо переносилось пациентами, проходило без видимых фармакокинетических взаимодействий с иммуносупрессивной терапией и риска мочевой инфекции. ИНГЛТ-2 могут улучшать гликемический и гемодинамический контроль, антропометрические показатели, повышать уровень эритроцитов и снижать гиперурикемию у стабильных реципиентов ПТ с ПТСД по сравнению с контролем. ИНГЛТ-2 можно рассматривать как вариант лечения пациентов с ПТСД.
В то же время требуются дополнительные масштабные, долгосрочные исследования пациентов после трансплантации.



