Введение
Вторичный гиперпаратиреоз (ВГПТ) считается распространенным клинически значимым осложнением прогрессирующей хронической болезни почек (ХБП) и является компонентом хорошо известного синдрома минерально-костных нарушений при ХБП (МКН-ХБП), который включает нарушения минерального обмена, метаболизма костной ткани, кальцификацию сердечно-сосудистой системы и мягких тканей. ВГПТ характеризуется увеличением скорости костного метаболизма, риска переломов, кальцификации сосудов и, что особенно важно, сердечно-сосудистой и общей смертности [1, 2].
Результаты крупных международных наблюдательных исследований показали независимую связь между уровнем концентрации паратиреоидного гормона (ПТГ) более 600 пг/мл с повышенным риском общей и сердечно-сосудистой смертности, а также госпитализаций, связанных с сердечно-сосудистыми заболеваниями. Более того, поддержание показателей костного метаболизма, включая уровень ПТГ, в пределах рекомендованного диапазона, служит значимым предиктором выживаемости и сохранения качества жизни пациентов, получающих лечение программным гемодиализом (ПГД) [2, 3].
Современные принципы терапии ВГПТ, доказанные эффекты
Витамин D и его активные метаболиты в течение нескольких десятилетий являлись краеугольным камнем терапии ВГПТ, эффективно подавляя секрецию ПТГ у большинства пациентов, но в то же время способствуя повышению всасывания кальция и фосфатов в кишечнике, что могло быть серьезным препятствием для их использования в ряде случаев [4].
Кроме того, аналоги витамина D повышают циркулирующие уровни FGF-23, связанного с неблагоприятными клиническими исходами, такими как прогрессирование ХБП, кальцификация артерий, гипертрофия миокарда левого желудочка, сердечно-сосудистые заболевания и увеличение смертности [5–7]. Напротив, фармакологическое вмешательство, которое позволит снизить уровень FGF-23, должно быть перспективным в отношении положительного влияния на эти значимые клинические исходы.
Необходимость преодоления недостатков ВДРА стимулировала поиски альтернативных препаратов, способных влиять на секрецию ПТГ через иные патогенетические механизмы, в частности через прямое воздействие на кальций-сенсорные рецепторы (CaSR) клеток ПЩЖ. В результате появился новый класс препаратов – кальцимиметиков. Первое поколение кальцимиметиков не получило клинического применения из-за выявленных существенных неблагоприятных явлений при его применении. Первым одобренным для клинического применения (но относящимся ко второму поколению) кальцимиметиком стал цинакальцет (ЦК).
В настоящее время международными и национальными клиническими рекомендациями одобрены следующие варианты лечения ВГПТ: снижение потребления фосфатов с пищей (в частности, рекомендуется потреблять меньше полуфабрикатов с высоким содержанием фосфатных пищевых добавок и отдавать предпочтение растительному белку) и применение фосфат-связывающих препаратов (ФСП); ингибирование синтеза и секреции ПТГ путем приема кальцимиметиков или активаторов рецепторов к витамину D (ВДРА), а также их сочетанного применения. При неэффективности фармакотерапии в рефрактерных случаях рассматривают вариант хирургического удаления паращитовидных желез (паратиреоидэктомия – ПТЭ) [8, 9].
До сих пор отсутствуют проспективные РКИ, которые показали бы результативность лечения ФСП или ВДРА в отношении неблагоприятных исходов ВГПТ, хотя в них доказано статистически достоверное положительное влияние этих препаратов на многие патогенетически значимые маркеры МКН-ХБП.
За 15 лет и более, прошедших с момента регистрации ЦК, в нескольких РКИ продемонстрирована его эффективность в отношении улучшения биохимического контроля МКН-ХБП, снижения синтеза, секреции ПТГ и потребности в ПТЭ, улучшения структуры костной ткани и уменьшения риска переломов. Кроме того, препарат был эффективным даже при стойком и рецидивирующем гиперпаратиреозе после ПТЭ, не сопровождался внекостной кальцификацией и ослаблял развитие кальцификации при сочетанном применении с ВДРА [10- 12].
В крупном рандомизированном плацебо-контролируемом двойном слепом исследовании EVOLVE участвовали 3883 пациента на диализе с ВГПТ. В группе ЦК в дополнение к стандартной терапии был достигнут более высокий контроль ВГПТ и низкий риск развития тяжелого гиперпаратиреоза, чем в группе плацебо. Составная первичная конечная точка (время до смерти, сердечно-сосудистого явления без смертельного исхода, в т.ч. инфаркта миокарда, госпитализации вследствие нестабильной стенокардии, сердечной недостаточности или периферического сосудистого явления в зависимости от того, какое событие наступит первым) без внесения поправок показала незначительное снижение в группе ЦК в популяции всех рандомизованных пациентов. При внесении поправки на дисбаланс исходных характеристик и несоблюдение режима лечения было продемонстрировано номинально значимое снижение частоты достижения составной первичной конечной точки. Кроме того, дальнейший запланированный вторичный анализ данных исследования EVOLVE показал значительное снижение риска ПТЭ и развития кальцифилаксии при применении ЦК [13–15].
В исследовании ADVANCE участвовали пациенты на ПГД с умеренным или тяжелым ВГПТ. По сравнению с лечением ВДРА в гибких дозах ЦК в комбинации с ВДРА в низкой дозе уменьшал прогрессирование кальцификации коронарной артерии и аортального клапана после 52 недель лечения. Данный результат наблюдался при оценке по объемной шкале; результату при оценке по шкале Агатстона слегка недоставало статистической значимости (P= 0,07). Рost hoc-анализ данных, полученных от пациентов, соблюдавших режим лечения, продемонстрировал значительное замедление прогрессирования кальцификации сердечно-сосудистой системы, даже при оценке по шкале Агатстона [16, 17].
Что касается метаболизма и гистологии костной ткани, после применения ЦК в течение 6–12 месяцев у 77 пациентов на ПГД снижались показатели резорбции при сохранении достаточно высокого костеобразования, о чем свидетельствовали результаты биопсии. В целом улучшались гистологические показатели костной ткани, а у значительного числа пациентов гистология костной ткани нормализовалась [11].
Цинакальцет как в качестве монотерапии, так и при сочетанной терапии с активными формами витамина D, позволяет добиваться улучшения контроля ВГПТ; однако для него характерна низкая приверженность пациентов лечению, что в долгосрочной перспективе может снижать эффективность контроля ВГПТ. Согласно данным литературы, частота несоблюдения режима лечения цинакальцетом варьируется от 45,6 % до 71%. Это наблюдалось не только в клинической практике, но и непосредственно в исследовании EVOLVE, в котором 62,0% пациентов, рандомизированных в группу цинакальцета, прекратили прием препарата во время исследования [18].
По данным ряда исследований показано, что низкая приверженность лечению при приеме ЦК была связана с высокой частотой возникавших у пациентов побочных эффектов со стороны желудочно-кишечного тракта (в основном тошнота и рвота). Еще одной причиной низкой приверженности лечению при приеме цинакальцета может быть масса пероральных препаратов, которые вынуждены принимать пациенты на диализе. По данным Y. Chiu et al., пациенты на ПГД в среднем принимают по 19 таблеток в день [19]. При этом более половины от количества таблеток, принимаемых в день пациентом, составляют препараты для лечения ВГПТ. Многие больные, которым было показано лечение ЦК и не было препятствий для его приема в виде плохой переносимости, тем не менее прекращали его прием или не принимали в достаточной дозе [20, 21].
Таким образом, повышение приверженности лечению не только влияет на здоровье пациентов, но и имеет важное экономическое значение. Низкая приверженность лечению, часто встречающаяся среди пациентов на диализе, влечет за собой повышение заболеваемости и смертности, а также повышение расходов на лечение [22, 23].
Поскольку ЦК вызывает гипокальциемию (ожидаемый эффект на основе результатов ранних клинических исследований) и желудочно-кишечные нежелательные явления (преимущественно тошноту и рвоту), длительное его применение рядом пациентов в условиях клинической практики оказалось проблематичным и ограничивало его эффективность. Отсутствие постоянного приема назначенного препарата многими пациентами, а также расширение границ целевого диапазона ПТГ, предложенное в Руководстве KDIGO (что зачастую приводит к запаздыванию начала терапии), может объяснить, почему частота ПТЭ не снизилась за последние годы во многих странах, в т.ч. в США [24]. Действительно, несмотря на то что в Руководстве KDIGO есть оговорка, согласно которой терапия ВГПТ может быть начата при нарастающей динамике уровня ПТГ по результатам повторного исследования даже в интервале от 300 до 600 пг/мл, но по последним данным DOPPS [2], в клинической практике терапию начинают только после того, как уровень ПТГ превысил 600 пг/мл. Особняком в этом отношении стоит Япония, в которой наблюдается устойчивое снижение частоты ПТЭ [25], возможно связанное с более низким целевым интервалом ПТГ и более ранним началом терапии в соответствии с Национальными клиническими рекомендациями. Поскольку ПТЭ часто сопровождается субоптимальными и/или краткосрочными результатами, требующими повторных хирургических вмешательств [26, 27], крайне важно предотвратить развитие тяжелого рефрактерного ВГПТ, исключающего любое лечение кроме ПТЭ. Потенциал нового внутривенного кальцимиметика этелкальцетида (ЭК) в предотвращении ПТЭ представляется многообещающим благодаря возможности исключить недостаточную приверженность терапии. [28].
Кальций-сенсорные рецепторы (CaSR) как точка приложения терапии ВГПТ.
Влияние цинакальцета и этелкальцетида на CaSR
CaSR является G-протеин-ассоциированным рецептором класса С, состоящим из 1085 аминокислот. Внеклеточная часть рецептора включает 2 домена из 613 аминокислот. Первый образован двумя субдоменами (долями), на границе которых формируются два лиганд-связывающих углубления, по строению и действию напоминающих растение «венерина мухоловка» захлопывающееся при активации (присоединении лиганда). Поэтому домен обозначается VFT (аббревиатурой английского «Venus flytrap»). Между VTF-доменом и мембраной клетки располагается домен с высоким содержанием цистеина (cysteine-related, CR). Трансмембранный домен состоит из 250 аминокислот, внутриклеточный домен – из 222 аминокислот и оканчивается карбоксильной группой [29]. Активация и супрессия СaSR осуществляются массой эндогенных и экзогенных модуляторов (как прямых агонистов и антагонистов, связывающихся преимущественно с типичными местами VFT домена, так и аллостеричных, связывающихся с другими участками VFT или трансмембранного домена). Известно как минимум о пяти таких участках с разным аффинитетом к модуляторам, но предполагается, что это не полный список. CaSR широко экспрессирован как в тканях и органах, напрямую включенных в гомеостаз Са, так и в тканях, имеющих другие функции. К эндогенным и экзогенным агонистам относятся ионы кальция, магния, цинка, железа, стронция и некоторые другие двух- и трехвалентные ионы, а также полиамины (наличие множества положительных зарядов делает эти структуры эффекту частично схожими с высокими концентрациями кальция или магния). Эндогенные и экзогенные аллостерические модуляторы включают L-аминокислоты, гамма-глютамилпептиды (глютатион и его аналоги), фосфат (который обладает негативным аллостерическим эффектом). Кроме того, негативное модулирующее влияние на CaSR оказывает ацидоз (высокий уровень ионов водорода) и гипернатриемия (через повышение осмолярности плазмы).
CaSR является одним из ключевых участников поддержания гомеостаза кальция. Расположенные на клетках ПЩЖ рецепторы реагируют на концентрацию Са в крови. Set-point для полумаксимального ингибирования секреции ПТГ находится на нижней границе нормального диапазона концентрации ионизированного Са в сыворотке (1,1 ммоль/л). Но паратиреоидные CaSR не включены в защитные реакции при гиперкальциемии. Эту функцию осуществляют CaSR в толстом восходящем отделе петли Генле, усиливая экскрецию кальция.
CaSR широко экспрессированы как на апикальной, так и на базалатеральной мембране клеток канальцев: мониторируя концентрацию Са как в плазме, так и в моче, они влияют на конечную композицию ультрафильтрата и его ацидификацию. Парафолликулярные С-клетки щитовидной железы также снабжены CaSR, которые промотируют секрецию кальцитонина при повышении уровня сывороточного Са. Кальцитонин снижает поступление Са в кровь в основном за счет подавления резорбции кости. В костной ткани CaSR расположены на остеобластах, остеоцитах, остеокластах и на некоторых хондроцитах. Предполагается их участие в стимулировании пролиферации этих клеток в зависимости от уровня Са.
Однако функция CaSR не ограничивается только участием в минеральном и костном метаболизме. Они также расположены на кератиноцитах и играют существенную роль в обеспечении барьерных функций кожи. В кишечнике CaSR выступают как нутриент-сенсоры по отношению не только к Са и Мg, но и к L-аминокислотам, дипептидам и полипептидам. Они вовлечены в выделение желудочной кислоты, секрецию гормонов, абсорбцию нутриентов, поддержание гомеостаза кишечной жидкости, дифференциацию и пролиферацию кишечного эпителия, проводимость нервной системы ЖКТ, поддержание микробиоты. Рецепторы на ациноцитах поджелудочной железы участвуют в регуляции секреции пищеварительных ферментов, а на бета-клетках – в регуляции секреции инсулина и гомеостазе глюкозы. Их присутствие на секретирующих клетках молочной железы обеспечивает достаточную концентрацию кальция в материнском молоке. В гладкомышечных клетках и эпителии бронхов CaSR вовлечены в процессы воспаления и гиперактивности этих клеток при обструктивных заболеваниях легких. Значительная плотность рецепторов определяется на гладкомышечных клетках сосудов, эндотелии и периваскулярных нервах. Активация CaSR в эндотелии ведет к гиперполяризации мембраны, что сопровождается освобождением оксида азота и вазодилатацией. Напротив, активация CaSR в гладкомышечных клетках сосудов приводит к вазоконстрикции. Эти данные свидетельствуют о заинтересованности CaSR в регуляции тонуса сосудов. CaSR широко представлены в клетках структур мозга, причем их присутствие меняется с возрастом, что свидетельствует о важности активации этих рецепторов в процессе дифференциации нейронных и глиальных клеток [30].
Имеющиеся на сегодняшний день данные о CaSR позволяют предполагать, что воздействие кальцимиметиков может иметь существенно более широкое воздействие на организм, чем только влияние на минеральный и костный метаболизм. Не исключено, что подобно ВДРА, кальцимиметики обнаружат множество плейотропных эффектов при дальнейших исследованиях.
Все кальцимиметики действуют на СаSR как положительные аллостерические модуляторы. ЦК, будучи малой молекулой, присоединяется к трансмембранному домену рецептора. ЭК является крупной молекулой, имеет молекулярный вес 1048 Да, состоит из остова в 7 D-аминокислот и L-цистеина, присоединенного к D-цистеину через дисульфидный мостик с противоположного от NH2 конца остова. ЭК активирует CaSR, обменивая L-цистеин на цистеин 482 в цепочке VFT домена рецептора. Это место присоединения отличается как от того, к которому присоединяется Са, так и от места присоединения ЦК. Присоединение кальцимиметиков к CaSR модулирует третичную структуру CaSR и увеличивает его чувствительность к ионизированному Са. Это приводит к снижению установленной величины (set-point) для системного гомеостаза Са (гомеостаз достигается при более низкой концентрации Са), секреция ПТГ подавляется и, соответственно, снижаются уровни ПТГ и Са в плазме крови. В меньшей степени наблюдается снижение уровня фосфатов [31, 32].
Фармакокинетика (ФК) и фармакодинамика (ФД) этелкальцетида
Несмотря на одинаковую точку приложения ЦК и ЭК (CaSR), они различаются в отношении структуры, способа введения, длительности периода полувыведения и потенциала лекарственных взаимодействий с участием системы цитохрома P-450.
В отличие от ЦК, который метаболизируется энзимами CYP3A4, CYP2D6, CYP1A2 системы CYP450 и может взаимодействовать с другими препаратами, метаболизируемыми теми же ферментами (например, трициклическими антидепрессантами, некоторыми антиаритмическими препаратами – флекаидином, амиодароном), ЭК, за счет D-конфигурации аминокислот, составляющих его молекулу, не подвергается ферментативному распаду под действием протеолитических ферментов и не взаимодействует с изоферментами CYР450. ЭК подвергается биотрансформации, вступая в реакцию дисульфидного обмена с эндогенными тиолами. В результате происходит обратимое образование конъюгатов альбумин-пептид. Данные конъюгаты не выводятся при диализе, поскольку их молекулярная масса составляет 67 кДа. В присутствии L-цистеина происходит обратный дисульфидный обмен с образованием ЭК, при этом прямая реакция (образование конъюгата) протекает быстрее, чем обратная. ЭК обладает высокой внутрииндивидуальной вариабельностью в распределении и элиминации, достигающей 60–70%, что объясняет вариабельность ФК у пациентов и необходимость титрации для достижения лечебной дозы. В исследованиях, проверявших иммуногенность, частота образования антител к ЭК составила от 1,4 до 7,1%, однако появление антител не влияло на ФК и ФД и безопасность препарата [32–35].
Как и ЦК, ЭК приводит к быстрому дозозависимому снижению концентрации ПТГ, Са, Р и FGF-23 и, в отличие от ЦК, способен активировать CаSR даже в отсутствие Са, что свидетельствует о наличии у него дополнительной функции прямого агониста CаSR. ЭК практически полностью выводится почками посредством клубочковой фильтрации. Вследствие этого период полувыведения ЭК из плазмы значительно повышается при снижении функции почек, а у пациентов с терминальной стадией почечной недостаточности его эффективный период полувыведения составляет от 3 до 5 дней. Длительность снижения уровня ПТГ при однократном в/в введении этелкальцетида пациентам на ПГД достигает 72 часов, что позволяет вводить его 3 раза в неделю. Поскольку при ПГД происходит довольно быстрая элиминация препарата, рекомендуется вводить его при отключении от аппарата [36, 37].
Данные исследований эффективности и безопасности этелкальцетида
Результаты предрегистрационных рандомизированных исследований (РКИ) II и III фаз, оценивавшие эффективность и безопасность ЭК по сравнению с плацебо и с ЦК подробно описаны в статье Н.А. Михайловой (2018) [38]. Они продемонстрировали несколько большую эффективность ЭК относительно ЦК в отношении ключевых параметров ВГПТ – ПТГ и FGF23, но при этом, как правило, возрастала и частота гипокальциемии. Частота другого распространенного нежелательного явления (НЯ) при терапии кальцимиметиками (тошноты и/или рвоты) не различалась в группах ЭК и ЦК [39–42]. Вторичный анализ сравнительных исследований III фазы ЭК и плацебо и ЭК и ЦК подтвердил более выраженное снижение FGF23 на фоне терапии ЭК не только по сравнению с плацебо (-56% vs +2%), но и с ЦК (-68% vs 41%). Следует отметить, что в группе ЭК дозы витамина Д были выше и это могло частично нивелировать результаты в группе ЭК [43]. На основании этих исследований и данных о ФК и ФД в таблице представлены основные различия между ЭК и ЦК.
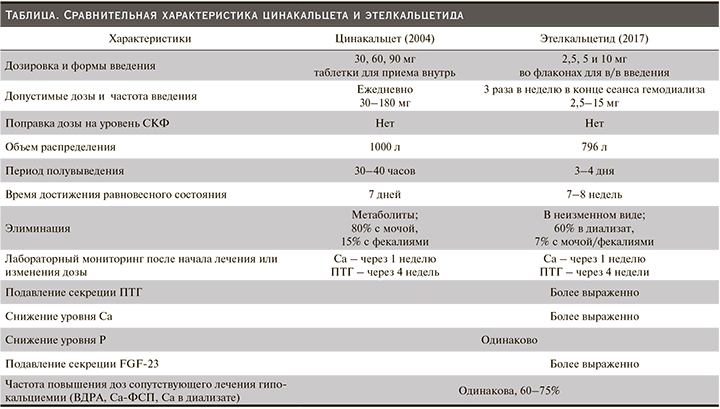
В течение двух последних лет возрос интерес к результатам использования различных препаратов в реальной клинической практике. Эти исследования не предполагают тщательного отбора пациентов и изменений дозировок в соответствии с жестким протоколом РКИ, но дают представление о реакции на препарат общей популяции пациентов, его получающих. Ниже приводим результаты таких исследований, касающихся ЭК.
В исследовании D Russo et al. (2019) [44] из 23 диализных центров были отобраны 168 (14%) пациентов, длительно получавших ЭК. Единственным фактором, исключавшим участие пациента в исследовании? было удлинение интервала QT. Треть пациентов изначально получала ЭК, остальные переведены на ЭК с ЦК с целью лучшего контроля ВГПТ (63% из пациентов с хорошей переносимостью ЦК не достигли целевого уровня ПТГ), снижения побочных эффектов (диспептические расстройства были у 61 из 112 пациентов? ранее получавших ЦК, 24 вынуждены были прекратить прием ЦК из-за них) и для увеличения приверженности терапии (4 пациента). Еще 19 пациентов были переведены на ЭК для снижения пероральной таблетированной нагрузки. Доза ЭК колебалась от 7,5 до 45 мг/нед (медиана – 15 мг/нед). Средний исходный уровень ПТГ 636 пг/мл снизился к концу исследования до 357 пг/мл. Число пациентов, достигших референсного интервала значений ПТГ, возросло с 27 до 63%. Нежелательные явления со стороны ЖКТ (боль в эпигастрии, диарея и тошнота) на ЭК возникали в 3–4%, в отличие от ЦК, на котором количество НЯ со стороны ЖКТ составило 53%. Шесть пациентов вынуждены были прекратить введение ЭК в сроки от 30 до 90 дней с начала терапии из-за клинически значимой гипокальциемии, 1 пациент отказался от ЭК к 180-му дню исследования из-за тошноты и рвоты. Авторы заключают, что в реальной клинической практике нежелательные гастроинтестинальные эффекты на ЭК возникают значительно реже, чем в РКИ (3–4 против 10–12%) и несравнимо реже, чем на терапии ЦК (3–4 против 53%). Гипокальциемия ниже 7,9 мг/дл (1,97 ммоль/л), т.е. границы, за которой начинает повышаться смертность [45], не зафиксирована у 80% пациентов за все время исследования, несмотря на то что никому из пациентов не повышались дозы ВДРА или Са-содержащих ФСП.
В работе DA Bushinsky et al. (исследование OLE, 2019) [46] 682 пациента, ранее участвовавших в 3 РКИ, наблюдались еще 52 недели. Все пациенты независимо от того, получали ли они плацебо, ЦК или ЭК во время участия в РКИ, далее получали только ЭК. Снижение ПТГ >30% от исходного уровня отмечено у 68% пациентов, целевой интервал достигнут у 56%. Особое внимание в этом исследовании уделено отслеживанию НЯ. Они были отмечены у 89,8% пациентов. В большинстве (43,3%) случаев это была гипокальциемия, хотя симптоматической она была только в 3,7% случаев. Другие наиболее частые НЯ включали диарею (10,8%), рвоту (10,4%) и тошноту (9,6%). Эти данные не отличаются от плацебо-контролируемых РКИ. Частота НЯ, связанных с гипокальциемией (удлинение QT, желудочковые аритмии, мышечные судороги), наблюдались менее чем у 1% пациентов. Авторы отмечают, что поскольку процессы реполяризации в сердечной мышце и влияние на калиевые каналы связаны с гипокальциемией, а не с прямым воздействием ЭК [47], мониторинг уровня Са служит лучшей профилактикой нарушений проводимости и ритма. Прервать терапию ЭК из-за НЯ были вынуждены менее 5% пациентов. Сопутствовавшая терапия, направленная на поддержание уровня Са, состояла из кальцитриол или ВДРА у 81,8% пациентов, 67,5% дополнительно принимали Са-содержащие ФСП.
Несколько исследований посвящены проблеме перевода пациентов с терапии ЦК на ЭК в связи с недостаточной эффективностью перового. У части пациентов с тяжелым ВГПТ ЦК может оказаться неэффективным при развитии аденомы ПЩЖ или при нодулярной гиперплазии ПЩЖ, когда в железе преобладают клетки с низкой экспрессией CaSR, нечувствительные к кальцимиметикам. Например, в ретроспективном обсервационном исследовании среди 1268 пациентов, находившихся на ПГД в течение 2005–2015 гг. в 7 центрах, и получавших цинакальцет в среднем в течение 21±12 месяцев 41% имели неконтролируемый ВГПТ (уровень ПТГ девятикратно больше верхней границы нормы) [48]. Но низкая эффективность ЦК в ряде случаев может быть обусловлена отсутствием или недостаточной приверженностью к лечению. Согласно данным ВОЗ, приверженность лекарственному лечению среди пациентов с хроническими заболеваниями не превышает 50% [49]. Перевод на терпию ЭК позволяет дифференцировать истинную резистентность к ЦК от такой псевдорезистентности.
В недавно опубликованном исследовании M.D. Arenas et al. (2020) [50] приверженность к терапии ЦК определяли по опроснику SMAQ, разработанному для пациентов с ВИЧ-инфекцией и опробованному с положительным результатом пациентами на ПГД [51]. Для участия в исследовании отобраны 25 пациентов (10 приверженных и 15 не приверженных) с неудовлетворительным контролем ВГПТ. Пациенты были переведены на терапию ЭК через неделю (отмывочный период) по окончании приема ЦК. Дополнительным подтверждением неприверженности 15 пациентов было отсутствие повышения ПТГ через неделю после отмены ЦК. У тех, кто был оценен как приверженный терапии, уровень ПТГ несколько повысился к началу терапии ЭК. Начальная доза ЭК составила 2,5 мг/диализ в комбинации с парикальцитолом (ПК) 1–2 мкг/диализ (в зависимости от уровня кальция). Дозы ЭК и ПК титровались ежемесячно в зависимости от уровней ПТГ и Са. Лечение продолжалось 8 месяцев. У неприверженных пациентов ПТГ снизился с 818 до 367 пг/мл, у приверженных – с 496 до 228. Число пациентов, достигших целевого интервала, увеличилось с 28 до 58%. Частота гипокальциемии менее 2,1 ммоль/л увеличилась с 8% до 40%, хотя и оставалась бессимптомной. Достижение целевого интервала уровня фосфатов увеличилось с 40 до 65%. Еще одна сходная публикация была представлена испанскими исследователями [52]. В проспективное одноцентровое обсервационное исследование были включены 29 пациентов с ВГПТ на онлайн-ГДФ-программе 3 р/нед. Пациенты получали ЦК, но 38%, согласно опроснику, были не вполне привержены лечению, остальные имели ПТГ выше 300 пг/мл (в среднем 473 пг/мл). После перевода на ЭК пациенты наблюдались 6 месяцев, в течение которых у всех ПТГ снизился примерно в 2 раза.
В качестве иллюстрации высокой эффективности ЭК в лечении тяжелого ВГПТ, резистентного к терапии ЦК, можно привести публикацию уникального клинического случая сочетания ВГПТ и беременности [53]. Молодая пациентка, страдавшая ТХБП в исходе люпус-нефрита, поступила на ПГД в возрасте 21 года. Через год пребывания на ПГД у пациентки наступила первая беременность, которая протекала благоприятно и закончилась родоразрешением на сроке 32 недели. Уровень ПТГ в то время был в пределах 150 пг/мл. В течение следующих 3 лет у пациентки развился тяжелый ВГПТ с повышением ПТГ до 1500 пг/мл вследствие отказа принимать какие-либо ФСП, ВДРА или кальцимиметики. В возрасте 25 лет пациентка забеременела повторно. В качестве терапии ВГПТ пациентка получала кальцитриол и холекальциферол, что позволило стабилизировать ПТГ на уровне 500–800 пг/мл, но после 20-й недели гестации у пациентки начали последовательно обнаруживаться бурые опухоли в правой бедренной кости, вертлужной впадине, большом вертеле. Родоразрешение произведено кесаревым сечением на сроке 36 недель, ребенок здоров. В течение 5 недель пациентка кормила грудью и отказывалась от медикаментозной терапии. Уровень ПТГ быстро увеличился до 2000 пг/мл. Терапия ЦК в нарастающих дозах от 30 до 90 мг/сут в сочетании с кальцитриолом 0,5 мкг/сут не увенчалась успехом, напротив, появлялись все новые и новые бурые опухоли в правой большеберцовой кости, лонном сочленении, нескольких ребрах, черепе и большом пальце левой кисти. Перевод на терапию ЭК в дозе 15, а затем 30 мг/нед позволил снизить ПТГ до 200 пг/мл, избавиться от болевого синдрома, произвести несколько хирургических вмешательств по удалению бурых опухолей и полностью восстановить мобильность пациентки. По данным DXA, состояние минеральной плотности костей скелета соответствовало умеренной остеопении.
Эти исследования демонстрируют, что ЭК эффективен как при истинной, так и при псевдорезистентности к ЦК
Помимо наличия биохимического влияния на минерально-костный обмен в экспериментах на грызунах с уремией показано, что этелкальцетид снижает пролиферацию клеток ПЩЖ и повышает в них экспрессию рецепторов CаSR, ВДРА и FGFR1 [54]. Объемных клинических исследований влияния на размеры гиперплазированных ПЩЖ пока не было, но публикация клинического случая подтверждает экспериментальные данные [55]. У 59-летнего мужчины с ТХБП в исходе диабетической нефропатии через 10 лет пребывания на ПГД развился ВГПТ. Уровень ПТГ достиг 783 пг/мл и не поддавался снижению на фоне лечения селективным ВДРА максакальцитолом. Максакальцитол был заменен на ЦК в дозе 75 мг/сут, однако контроль ВГПТ был недостаточным и через 4 года при УЗИ было обнаружено три гиперплазированных ПЩЖ. Пациент был переведен на ЭК в дозе 30 мг/нед. Размеры наибольшей из ПЩЖ, составившие в начале терапии 1142 мм3, через 3 месяца уменьшились до 629,7, а еще через 6 – до 33,6 мм3. Размеры двух других ПЩЖ и уровень ПТГ также нормализовались.
Интерес к влиянию ЭК на сердечно-сосудистую систему высокий, и первые экспериментальные и клинические данные, полученные в последние три года, можно назвать многообещающими. Так, в экспериментах на крысах с уремией и ВГПТ ЭК вызывал значительное снижение уровня ПТГ, а также содержания Са в аорте, предотвращая кальцификацию медиального слоя аорты. Данные эффекты могут объясняться прямым действием ЭК на клетки эндотелия и гладких мышц кровеносных сосудов, в которых присутствует экспрессия CaSR, или на FGF-23-чувствительный путь [56].
Давно отмечена взаимосвязь между тяжестью ВГПТ и выраженностью артериальной гипертензии (АГ) у пациентов на ПГД. Предполагается, что после снижения уровня ПТГ из сосудистой стенки удаляются излишки кальция, уменьшается ригидность сосудистой стенки и АД снижается [57]. Еще одним описанным механизмом является прямое воздействие ПТГ на ПТГ2-рецепторы, расположенные в стенке сосуда, стимулирующее выработку коллагена [58]. Имеют место и проатеросклеротические воздействия ПТГ, такие как стимуляция экспрессии рецепторов к конечным продуктам гликозилирования (КПГ), повышение продукции цитокинов моноцитами-макрофагами и ИЛ-6 Т-клетками, что вызывает воспаление эндотелия и повышение жесткости сосудистой стенки. ПТГ способен влиять на контрактильную и хронотропную активность кардиомиоцитов. Высокий уровень ПТГ провоцирует развитие ГМЛЖ со снижением сократимости и увеличением ЧСС. К тому же повышение FGF23 при ВГПТ приводит к задержке Na через повышение экспрессии Na/Cl котранспортера в дистальном извитом канальце, создавая предпосылки для натрий-зависимой АГ. Все эти эффекты частично обратимы при нормализации ПТГ и уровня Са, что сопровождается снижением как систолического, так и диастолического АД. В исследовании EVOLVE показано, что снижение АД наблюдалось только в группе ЦК [59]. В сравнительных исследованиях ЦК и ЭК первичные и вторичные конечные точки касались эффективности терапии ВГПТ и частоты НЯ (гипокальциемия и диспепсия), влияние на АД не изучалось. Однако, возможно, более благотворное влияние на АГ демонстрируется клиническим случаем, в котором у молодого пациента на фоне терапии умеренного ВГПТ, леченного небольшими дозами ЦК, внезапно стал быстро нарастать уровень ПТГ (до 1200 пг/мл). Параллельно возникла резистентная к медикаментозной и диализной терапии АГ (190/100). Перевод пациента на терапию ЭК в дозе 15 мг/нед, а затем 22,5 мг/нед позволил в течение 8 месяцев нормализовать уровень ПТГ и одновременно с этим уровень АД [60].
Исследования на животных предоставили первые данные в поддержку гипотезы, что ЭК может быть эффективным в лечении почечной остеодистрофии. В экспериментах на крысах с удаленной почкой, у которых развился ВГПТ, ЭК ослаблял эффекты, вызванные ВГПТ (повышение пористости компактного вещества кости, дефекты минерализации, фиброз костного мозга), и повышал прочность костной ткани [61]. Другое недавнее экспериментальное исследование [62] показало прямое анаболическое действие кальцимиметиков на кость через активацию CaSR на клетках кости, улучшающее как статические, так и динамические гистоморфометрические параметры, помимо опосредованного влияния через снижение секреции ПТГ и FGF23 и активации синтеза кальцитриола. Следует отметить, что в двух РКИ III фазы, сравнивавших ЭК и плацебо, было продемонстрировано, что через 26 недель лечения в группе ЭК улучшились маркеры метаболизма кости [42]. В японском исследовании ЭК терапии ВГПТ [63] параллельно снижению ПТГ улучшались показатели костного обмена: костно-специфическая щелочная фосфатаза и тартрат-резистентная кислая фосфатаза.
В этом году опубликован первый мета-анализ эффективности всех существующих кальцимиметиков в терапии ВГПТ [64] В мета-анализ включено 35 РКИ и квази-РКИ, проведенных с 2000 по 2018 г. РКИ касались сравнения трех кальцимиметиков – ЦК, ЭК и эвокальцета. Сравнивались все три кальцимиметика с плацебо, два новых кальцимиметика (внутривенный ЭК и пероральный эвокальцет) с ЦК, а также ЦК с стандартной терапией, ВДРА и ПТЭ. Сделать выводы о сравнительном влиянии на сердечно-сосудистые исходы не удалось из-за недостатка данных. В отношении снижения ПТГ наиболее эффективен ЭК, но у него и наибольшая частота гипокальциемии. Наименее эффективен эвакальцет. Частота тошноты и рвоты наиболее высока при применении ЦК, наименьшая – при применении эвакальцета. Авторы сделали вывод: при выборе кальцимиметика нужно опираться на подтвержденные эффекты: если у пациента тяжелый ВГПТ или отсутствует приверженность лечению – выбор следует сделать в пользу ЭК, при повышенной опасности гастроинтестинальных проблем – выбор в пользу эвакальцета, при достаточной приверженности и переносимости – выбор в пользу ЦК. Ориентироваться на возможное превосходство в предотвращении сердечно-сосудистой и общей смертности пока не представляется возможным.
Дополнительные предосторожности, о которых следует помнить при выборе терапии этелкальцетидом
Остается открытым вопрос о потенциальной опасности удлинения электрокардиографического QT-интервала на фоне терапии кальцимиметиками, в т.ч. и ЭК (и вероятно, связанного прежде всего с гипокальциемией), являющегося предиктором желудочковой аритмии и внезапной смерти в общей популяции и у пациентов на ПГД [65]. Нормой считается продолжительность QT не более 450 мс у мужчин и не более 470 мс у женщин. Данные о QT-интервале отсутствуют в публикациях результатов EVOLVE, но в предрегистрационных РКИ эффективности и безопасности ЭК ЭКГ производилась регулярно в течение всего исследования. Пролонгация QT-интервала в сравнительных исследованиях III фазы ЭК с плацебо соответствовала более 450‐480 мс примерно у 25% пациентов, более 480–500 мс – у 5,6% и более 500 мс – у 2,7%. Удлинение QT интервала на 30–60 мс по сравнению с исходным уровнем наблюдалась у 19,5% пациентов (в группе плацебо – у 8,0%), более 60 мс – у 2,4% (в группе плацебо 0,0%) [66]. Из этого следует, что надо быть особенно осторожным при сочетанном применении ЭК с другими препаратами, удлиняющими QT-интервал: антиаритмиками (флекаидином, амиодароном), антимикробными препаратами (азитромицин, ципрофлоксацин), антидепрессантами (циталопрам, пароксетин), антипсихотиками (галоперидол, рисперидон), противорвотными (метоклопрамид, ондансетрон) и пр.
Повышенного внимания требуют пациенты с сахарным диабетом (СД) на ПГД, число которых растет и достигает уже 30% в диализной популяции [67]. QT-интервал часто удлинен у пациентов с СД и служил фактором риска внезапной ночной смерти пациентов на инсулинотерапии, а также пациентов без СД с гиперинсулинемией [68]. Предположительно возможны два механизма удлинения интервала QT при терапии инсулином: прямое действие на клеточные мембраны с активацией Na/К-АТФазы и поступление экстрацеллюлярного калия в клетку [69, 70] и симпатическая стимуляция, индуцированная гипогликемией и гипокалиемией [71]. Поэтому у пациентов с СД на терапии ЭК следует тщательно мониторировать ЭКГ.
Поскольку ЭК обратимо ковалентно связывается с альбумином, гипоальбуминемия на фоне белково-энергетической недостаточности или нарушения функции печени могут увеличивать свободную фракцию ЭК и приводить к неожиданно быстрому падению ПТГ и гипокальциемии. Кроме того, связывание с альбумином может быть изменено при неферментативном гликировании протеинов у пациентов с СД по аналогии с сульфонилмочевиной [72]. Сахароснижающие препараты, рекомендованные пациентам на ПГД (глипизид, репаглинид), также связываются с белками сыворотки на 99% и могут вступать в конкурентные взаимоотношения с ЭК, повышая его свободную фракцию. В меньшей степени, но с альбумином связывается также экзогенный инсулин при подкожном введении и лираглютид (агонист рецептора глюкагон-подобного пептида 1).
В клинических рекомендациях KDIGO (2017) по лечению МКН-ХБП больше не рекомендуется поддерживать сывороточные концентрации Са у пациентов на диализе в пределах диапазона референсных значений. Вместо этого предлагается не допускать развития гиперкальциемии; кроме того, допускается наличие у пациентов слабой бессимптомной гипокальциемии, связанной с терапией кальцимиметиками, с целью недопущения чрезмерного приема Са [8] Но следует помнить, что чувствительность к гипокальциемии со стороны миокарда более выражена у пациентов с СД и описана индуцированная гипокальциемией декомпенсация предсуществовавшей хронической сердечной недостаточности [73]. Учтя гипокальциемический эффект этелкальцетида и связанные с ним изменения в проводимости миокарда, считаем, что начинать лечение этелкальцетидом можно при скорректированном сывороточном уровне Са ≥8,3 мг/дл (2,07 ммоль/л).
Заключение
Таким образом, ВГПТ характеризуется увеличением скорости костного метаболизма, риска переломов, кальцификации сосудов, а также сердечно-сосудистой и общей смертности. Цинакальцет — первый кальцимиметик, одобренный к клиническому применению, эффективно снижает уровень ПТГ и положительно влияет на биохимические показатели при минеральных и костных нарушениях у пациентов с ХБП. Тем не менее влияние цинакальцета на «жесткие» конечные точки требует уточнения.
В крупных, тщательно проведенных РКИ показано, что новый кальцимиметик третьего поколения этелкальцетид является эффективным средством для снижения уровня ПТГ и может использоваться нефрологами в качестве альтернативы аналогам витамина D и цинакальцету у пациентов с ВГПТ на ПГД.
Этелкальцетид превосходит цинакальцет в отношении снижения уровней ПТГ и FGF-23 у больных ТПН, но увеличивает частоту эпизодов гипокальциемии (эффект может быть более выраженным в начале лечения), однако в большинстве случаев решается применением сопутствующих методов лечения, таких как использование аналогов витамина D, пероральных фосфатсвязывающих препаратов на основе кальция, повышение концентрации кальция в диализате.
При введении этелкальцетида может увеличиваться длительность интервала QT и лечащий врач должен тщательно его контролировать. Следует измерять концентрацию кальция в сыворотке крови перед началом и во время лечения, и практикующие врачи должны знать о потенциальных эффектах удлинения интервала QT.
Этелкальцетид вводится в конце процедуры гемодиализа, что улучшает приверженность терапии и снижает количество принимаемых таблетированных препаратов. Однако, несмотря на внутривенное введение, этелкальцетид, как и цинакальцет, может вызывать симптомы со стороны желудочно-кишечного тракта.
Полагаем, терапия этелкальцетидом обеспечивает значительное преимущество в лечении ВГПТ за счет улучшения контроля уровней ПТГ и FGF-23, а также приверженности терапии. Тем не менее вопрос, приводит ли улучшение биохимического контроля к улучшению клинических исходов, таких как частота переломов костей, сердечно-сосудистая заболеваемость и смертность, требует уточнения в проспективных рандомизированных исследованиях.



