Введение
Хроническая болезнь почек (ХБП) – глобальная проблема мирового здравоохранения [1]. Пятилетняя выживаемость пациентов с терминальной стадией почечной недостаточности (ХПН) составляет около 35% и в значительной степени обусловлена высокой сердечно-сосудистой смертностью (ССЗ) [2]. Факторы риска ССЗ для пациентов с ХПН кроме традиционных представлены и такими факторами риска, как воспаление, анемия, нарушение минерального обмена [3]. Существует определенный дефицит информации о роли и стратификации биомаркеров, отражающих новые факторы риска, в развитии экстраренальных, в первую очередь сердечно-сосудистых, осложнений у пациентов с ХБП, особенно 5Д-стадии. Сравнительно недавно выявленные участники регуляции кальций-фосфорного гомеостаза [4], такие как FGF-23 (fibroblast growth factor, фактор роста фибробластов) и alpha-Klotho (α-Klotho) [5], могут быть недостающим звеном в каскаде патогенетических механизмов развития сердечно-сосудистых заболеваний у пациентов с ХПН [5]. FGF-23 представляет собой циркулирующий гормон, секретируемый преимущественно остеоцитами и остеобластами, связывающийся в почечных канальцах с 1сFGF-рецептором и Klotho-корецептором [6]. У пациентов с ХБП в сыворотке крови повышается уровень FGF-23 в ответ на ретенцию фосфатов, в то время как концентрация α-Klotho уменьшается [7]. Повышенные уровни FGF-23 ассоциированы с неблагоприятными кардиоваскулярными исходами, но неизвестно, обусловлено ли это собственно кардиотоксическим действием данного фактора или дефицитом α-Klotho. Результаты исследования, изучающие взаимосвязь FGF-23, α-Klotho и морфофункциональные изменения сердца и сосудов, остаются противоречивыми [6, 8]. Белок α-Klotho, экспрессия которого происходит в эпителиальных клетках почечных канальцев, представлен двумя фракциями: мембраносвязанным и сывороточным α-Klotho, последний образуется из внеклеточной части мембраносвязанного α-Klotho [9]. Аlpha-Klotho играет важную роль в почечной экскреции кальция и фосфатов независимо от FGF-23 [10], а также выполняет защитную роль в процессе сосудистой кальцификации и развития оксидативного стресса, что было изучено в доклинических исследованиях [11]. Целью данной работы была оценка ассоциации между сывороточными уровнями α-Klotho, FGF-23 и структурными кардиоваскулярными изменениями у пациентов на хроническом гемодиализе.
 Материал и методы
Материал и методы
В исследование включены 83 пациента (45 мужчин и 38 женщин) с ХБП 5Д-стадии, получающие терапию хроническим гемодиализом из двух центров амбулаторного гемодиализа.
Средний возраст обследуемых пациентов – 53,7±14,9 года, минимальный индекс массы тела – 15,5, максимальный – 39,5 кг/м2, средний показатель Kt/V – 1,4±0,07. Доминирующими причинами ХПН стали хронический гломерулонефрит в 30,1% (25 человек) случаев, диабетическая нефропатия – в 18,1% (15), артериальная гипертензия и тубулоинтерстиуиальные нефриты – по 14,4% (12), аутосомно-доминантная поликистозная болезнь почек выявлена в 9,6% (8 пациентов). Процедуры гемодиализа проводились 3 раза в неделю по четыре-пять часов, в отношении всех больных использовалась бикарбонатная буферная диализная жидкость, содержащая 2–3 ммоль/л калия, 1,25–1,50 ммоль/л кальция, 0,75 ммоль/л магния, глюкозы 1–1,5 г/л и полисульфоновые диализные мембраны. Оценивались анамнестические, антропометрические данные, ежемесячно измеряемые параметры крови (общий анализ крови, концентрация в сыворотке крови креатинина, мочевины, кальция, фосфора, интактного паратиреоидного гормона, кальций-фосфорного произведения и других параметров), а также образцы плазмы крови, в которых определялись FGF-23 и α-Klotho методом иммуноферментного анализа. Забор плазмы для измерения концентрации FGF-23 и α-Klotho проведен перед началом очередной процедуры гемодиализа в пробирке с ЭДТА, после чего полученный материал центрифугировался и замораживался при температуре -20°С до получения необходимого количества исследуемых образцов с последующим измерением результатов.
Протокол исследования одобрен локальным независимым этическим комитетом ГБОУ ВПО РостГМУ. Все пациенты дали информированное согласие на участие в исследовании.
Эхокардиографическое обследование проведено на аппарате «PHILIPS HD 11» в В- и М-режиме, а также в допплеровском режиме исследования и включало следующие измерения: конечный диастолический размер (КДР, мм), конечный систолический размер (КСР) левого желудочка (ЛЖ, мм), толщину межжелудочковой перегородки в диастолу (мм), ЛЖ-толщину задней стенки (мм), диаметр левого предсердия (мм), диаметр корня аорты и ее восходящего отдела (мм, фракцию выброса (ФВ, %), фракцию укорочения (ФУ, %). Оценивалась диастолическая функция ЛЖ по результатам, полученным при исследовании трансмитрального диастолического кровотока в допплер-режиме (максимальная скорость раннего пика диастолического наполнения – Е (м/с), максимальная скорость трансмитрального кровотока во время систолы левого предсердия – А (м/с), отношение скоростей (Е/А) и др.).
Оценка кальциноза проведена по полуколичественной шкале оценки степени кальциноза для структур сердца, по шкале кальцификации брюшного отдела аорты с использованием ранее утвержденной системы классификации Kauppila, в котором степень кальцинозных отложений в брюшной аорты оценивается на каждом сегменте для аорты [12].
Использование препаратов, включая препараты кальция, фосфат-связыватели, аналоги витамина Д, антигипертензивные препараты, было указано.
С помощью компьютерной программы «STATISTICA 6.0» (StatSoft Inc., США) выполнен статистический анализ. Данные представлены в виде средних±стандартное отклонение (SD), использовались параметрические и непараметрические методы с оценкой корреляционной зависимости, линейный и нелинейный регрессионный анализы. Для выявления связи между признаками применяли корреляционный анализ Спирмана и Пирсона.
Результаты и их обсуждение
При обследовании пациентов в среднем уровень сывороточного FGF-23 составил 69,3±29,5 пкг/мл, вопреки привычным «катастрофическим» показателям данного параметра у пациентов с ХБП 5Д-стадией. На наш взгляд, это объясняется низкой продолжительностью диализной терапии (18,2±2 недели). FGF-23 – белок среднего размера [13] с молекулярной массой 32кДа, тогда как гемодиализная мембрана позволяет удалять токсины с небольшим диаметром молекулы (такие как мочевина, креатинин), поэтому элиминации FGF-23 не наблюдается [14, 15], в связи с чем уровень FGF-23 до и после процедуры гемодиализа (в отличие от гемодиафильтрации и гемодиализа с гемоперфузией) не изменяется [16]. Вероятнее всего, неспособность мембраны очищать кровь от данных молекул объясняет положительную корреляцию длительности гемодиализа и FGF-23. Тем же можно объяснить и столь невысокие цифры FGF-23 в нашей работе с длительностью гемодиализа 18,1±2 недели, тогда как в проанализированных нами исследованиях длительность диализа составила 31,7±39,8 месяца [16, 17]. Повышение уровня FGF-23 отмечено преимущественно у пациентов, у которых причиной развития ХБП был хронический гломерулонефрит (21% пациентов). S. Olivecrona, I. Gunnarsson опубликовали статью, в которой указана взаимосвязь IgА-нефропатии, FGF-23 и альбуминурии и предположено, что FGF-23 может непосредственно способствовать клубочковому повреждению ввиду того, что основным органом-мишенью для FGF-23 являются почки [18].
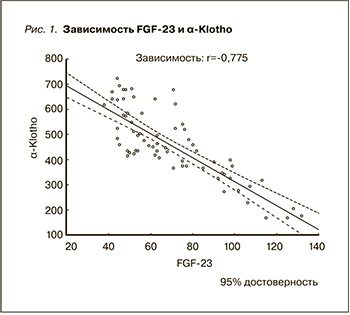
Что касается особенностей «взаимоотношения» FGF-23 и α-Klotho, то выявлена отрицательная корреляционная связь (r= -0,775, р<0,05) (рис. 1). FGF-23 уменьшает транскрипцию гена Klotho в почке, что обусловлено и FGF-23-опосредованным действием, и потерей массы действующих нефронов [19].
 При исследовании взаимосвязи уровня сывороточного FGF-23 с другими участниками кальций-фосфорного обмена было выявлено, что данный морфогенетический белок отрицательно коррелирует с ∆Са, полученной при вычислении разницы при измерении данного показателя двукратно с интервалом в месяц (r=-0,326, р<0,05), более корреляций данного показателя с лабораторными данными в нашем исследовании выявлено не было.
При исследовании взаимосвязи уровня сывороточного FGF-23 с другими участниками кальций-фосфорного обмена было выявлено, что данный морфогенетический белок отрицательно коррелирует с ∆Са, полученной при вычислении разницы при измерении данного показателя двукратно с интервалом в месяц (r=-0,326, р<0,05), более корреляций данного показателя с лабораторными данными в нашем исследовании выявлено не было.
Показатели α-Klotho соответствовали следующим значениям: 460,4±141,3 пкг/мл. До тех пор как были получены клинические данные о содержании в человеческой плазме α-Klotho, было показано, что в моче пациентов с ХБП уровень α-Klotho снижался с ранних стадий и существенно уменьшался по мере прогрессирования ХБП [19]. И на модели грызунов с ХБП уровни Klotho в плазме, моче и ткани почек уменьшались параллельно [20]. Для α-Klotho выявлена положительная корреляция с возрастом (r=-0,27, р=0,02), которая объясняется одной из функций α-Klotho в качестве возраст-модулирующего белка [21].
У пациентов с более низкими показателями α-Klotho (менее 460 пкг/мл) был выше в плазме процент насыщения трансферрина железом (33,37 против 27,6%; p=0,039), эффективная транспортная концентрация трансферрина (0,59 против 0,48 г/л, р=0,026) и β2-микроглобулин (23,6 против 19,9 мкг/мл, р=0,038). Увеличение процента насыщения трансферрина железом и эффективной транспортной концентрации трансферрина независимо от наличия или отсутствия анемии связано со свойствами трансферрина и его участием в активной иммунной реакции в ответ на длительное хроническое воспаление, т.к. оксидантный стресс способствует снижению α-Klotho [22]. Кроме того, α-Klotho коррелирует с уровнем-микроглобулина, β2-микроглобулина, что отражает не только локальное участие в повреждении почек, но и вовлечение в системный воспалительный процесс [23].
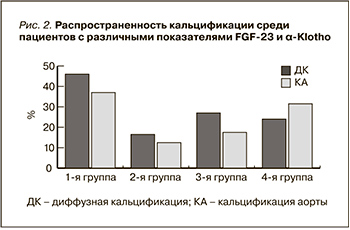
В среднем по группе у 40 пациентов наблюдалось повышение FGF-23 (>60,8 пг/мл), у 36 – снижение α-Klotho (<460 пг/мл), высокие значения FGF-23 и низкие α-Klotho – у 29 больных, изменения концентрации одного или другого показателя – у 46 пациентов, последние были определены в 1-ю группу.
У 25 пациентов уровни изучаемых факторов были в пределах нормальных значений, они были определены во 2-ю группу. Изменения концентрации только FGF-23 представили 3-ю группу, а α-Klotho – 4-ю.
Кальцификация клапанов сердца была выявлена у 34 (41%) пациентов, среди них кальцификация аортального клапана определена у 23 (27,7%) пациентов, митрального клапана – у 25 (30,1%), тогда как кальцификация этих двух клапанов определялась только у 12 (14,5%) пациентов. Среди обследуемых больных кальциноз клапанов сердца наблюдался в среднем в возрасте 61 года, тогда как кальцинаты не были сформированы у более молодых пациентов около 49 лет (Р<0,01).
Диффузная кальцификация исследуемых кардиоваскулярных структур (клапанов сердца и аорты, клапанов сердца или аорты) встречалась в 46% случаев в первой группе, тогда как 17,1% приходят на число пациентов с нормальными показателями данных морфогенетических белков (р=0,04). Среди пациентов с увеличенным FGF-23 диффузная кальцификация встречалась практически с той же частотой, что и у таковых с повышенными цифрами α-Klotho (24,3 и 27,1% соответственно) (рис. 2).
При этом кальциноз брюшного отдела аорты обнаружен в первой группе у 37,9%, только у 12,5% пациентов второй и в 17,4% третьей групп. Интересен тот факт, что в группе, где снижен только α-Klotho, выявлено, что кальциноз брюшного отдела аорты встречается среди множества пациентов в 31%, р=0,005. В исследовании in vitro K. Lim, T.S. Lu [11] была исследована роль α-Klotho в васкулярной патологии, показывая эндогенную экспрессию α-Klotho в сосудистых клетках гладких мышц [11]. Интересно, что именно ингибирование α-Klotho в гладкомышечных клетках аорты привело к ускоренной кальцификации этих клеток [11].
Кальцификация аортального клапана встречалась существенно чаще (48,6 против 27,1%, р=0,048), а митрального – 65% по сравнению с 35% (р=0,04) у пациентов первых двух групп; существенной разницы в кальцификации клапанов среди пациентов с повышенными показателями одного морфогенетического белка получено не было.
Далее мы исследовали ассоциацию риска развития кальцификации клапанного аппарата сердца и брюшного отдела аорты, используя логит-преобразование FGF23 и α-Klotho. Было установлено, что log(FGF23) по данным логит-регрессии не влияет на риск развития аортальной и клапанной кальцификации, другая ситуация с log(α-Klotho): выявлено, что, чем выше показатели α-Klotho, тем ниже риск развития аортальной кальцификации (р=0,02).
Таким образом, высокий уровень FGF-23 и низкий уровень α-Klotho служат маркерами кардиоваскулярной кальцификации. И изменение их концентрации – один из возможных факторов риска развития кальциноза структур сердца и аорты [24]. Тем не менее точная роль FGF-23, α-Klotho в прогрессировании сердечно-сосудистых заболеваний у больных на диализе остается дискутабельной.



